Is it Time to Re-Evaluate U.S. Pharmacopeia Standards? Exploring Y-site Pediatric Drug Administration Compatibility
October 17, 2023
Navigating the complex landscape of pediatric healthcare, particularly when it involves administering intravenous (IV) medication to children, brings about a unique set of difficulties. The task becomes even more daunting when dealing with critically ill patients who frequently require extensive IV drugs to maintain homeostasis.
To put it into perspective, an infant in a neonatal intensive care unit might receive an average of 8.5 IV drugs during a single admission, while children in a pediatric intensive care unit can receive a staggering number of up to 49 IV medications.1
Navigating the complex landscape of pediatric healthcare, particularly when it involves administering intravenous medication to children, brings about a unique set of difficulties. The task becomes even more daunting when dealing with critically ill patients who frequently require extensive IV drugs to maintain homeostasis. To put it into perspective, an infant in a neonatal intensive care unit might receive an average of 8.5 IV drugs during a single admission, while children in a pediatric intensive care unit can receive a staggering number of up to 49 IV medications.1
Due to the challenges associated with pediatric patients, medications are typically prepared and administered at different concentrations compared to adult patients. This is largely because infants have higher nutritional needs. However, this process is not without risks. Incompatible IV medications can produce particles, invisible to the naked eye. Recent studies have shown that infants undergoing multidrug IV therapies can potentially be exposed to up to 85,000 such subvisible particles each day.1
The impact of these particles on pediatric patients can be profound, leading to serious complications like pulmonary dysfunction, cardiovascular arrest, and multiorgan failure. Furthermore, these particles may negatively affect immune responses, adding another layer of risk. The danger of unintentionally administering these particles increases when co-administering untested medications for physical compatibility.1
For this reason, researchers set out to evaluate the physical intravenous Y-site compatibility of 29 combinations of medications at concentrations commonly used in pediatrics using both conventional and innovative techniques.1 In a study published in The Journal of Pediatric Pharmacology and Therapeutics, subvisible particle analysis was performed using Backgrounded Membrane Imaging (BMI), a high-contrast imaging technique on Aura. With the ability to test 96 samples in less than two hours, it’s a valuable tool for drug development.
Understanding Particulate Matter in Injectable Products
Pediatric patients often receive multiple IV medications via a Y-site connection, due to difficulties with securing reliable vascular access. However, this can lead to medication interaction and potential particle formation. This puts healthcare providers in a tough position—risking co-administering medications without compatibility data or dealing with the challenges associated with additional vascular access.1
Given the slow infusion rates used in pediatric patients, IV medications can interact with each other for extended periods before entering the bloodstream. Even with an inline filter, substantial mixing of medications can occur downstream of the filter, potentially leading to particle formation if the medications are physically incompatible.1
Traditional methods for assessing particulate matter in injectable products, as outlined by the US Pharmacopeia (USP), include light obscuration (LO) and microscopic particle count tests. However, newer techniques such as flow imaging (FI) and BMI provide more accurate identification of particles and better characterization of their morphology.1
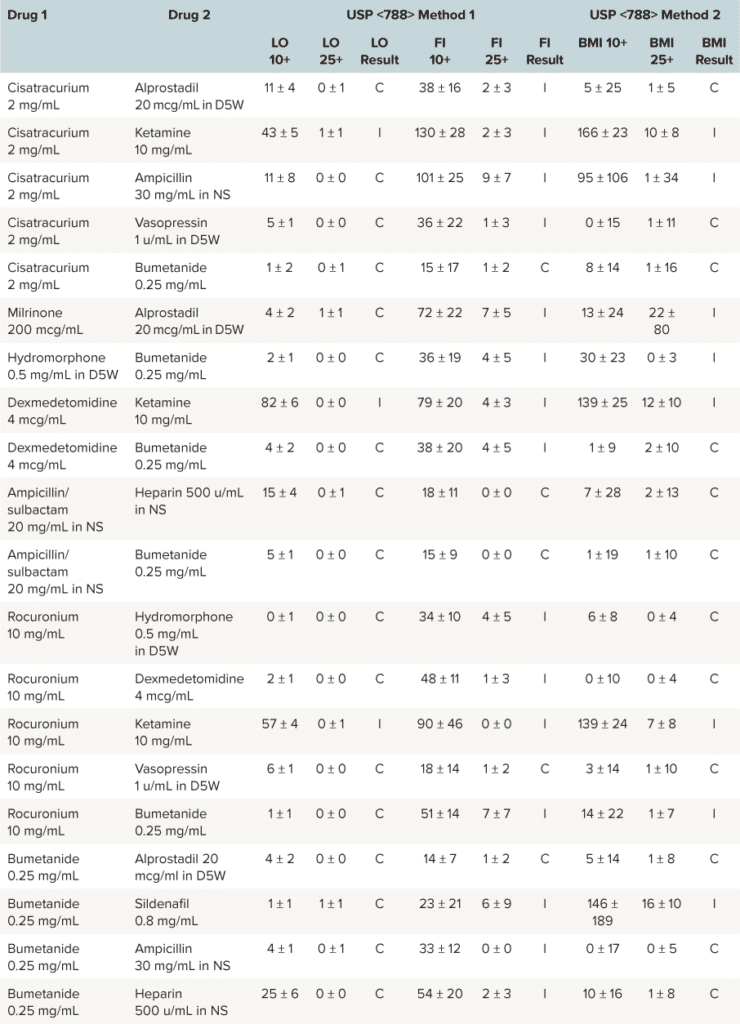
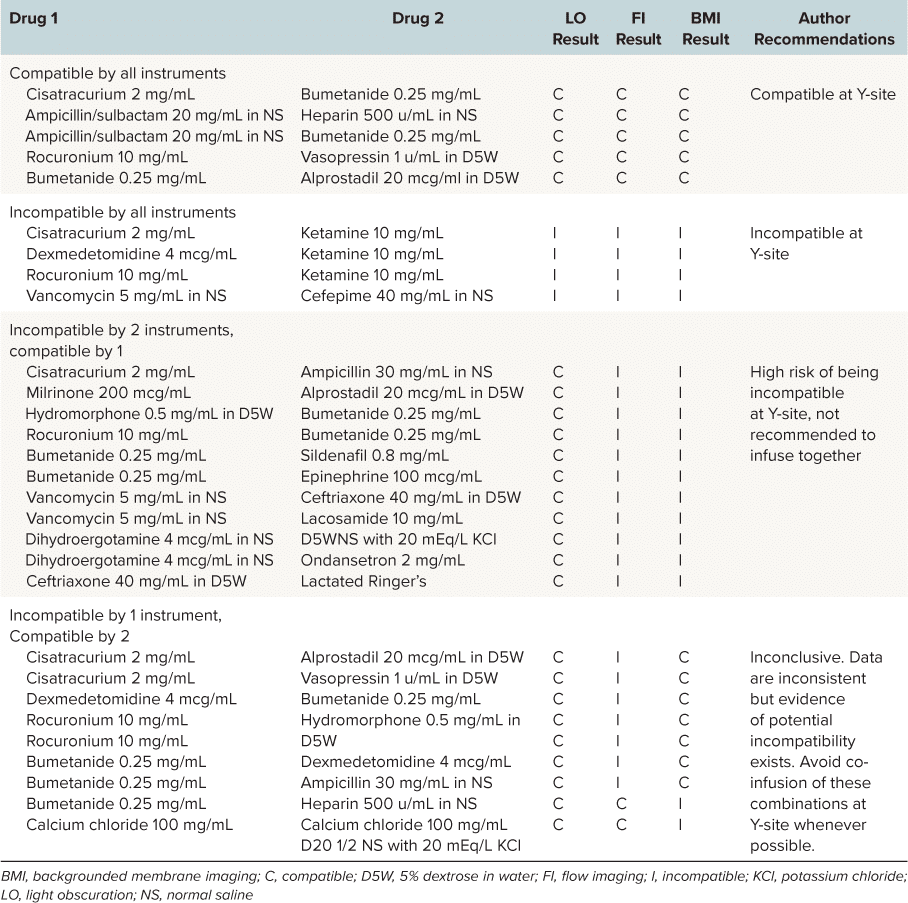
Subvisible Particle Analysis via BMI
Particles in high quantities or those with large diameters have the potential to be more devastating in small infants and children compared with adults, due to smaller pulmonary capillary size and relatively large fluid intake relative to their body weight. Because of the administration challenges specific to pediatric patients and the clinical risk associated with particle infusions, FI and BMI instrumental methodologies were used to assess USP <788> methods 1 and 2, despite a lack of precedent for either method in USP <788> guidelines. In this evaluation, these two methods demonstrated much higher accuracy for identifying particulates in solution when compared with LO.1
Applying USP <788> methods clinically to pediatric pharmacy practice brings to light clinical and analytical controversies. Are current LO and microscopy methods the most appropriate to use to test the compatibility of medication combinations? Additionally, are the current USP <788> particle count thresholds still applicable given the increased particle detection rates of instrumental methods now available? The USP chapter offers no explanation as to where the existing particle count thresholds originated, why method 1 has particle count limits that are essentially double that of method 2, or why large-volume parenterals are held to a different standard when compared with small-volume parenterals.1
Nevertheless, the team of researchers felt that results of incompatibility from any method used should preclude clinical use of a medicine combination for all patients, and for newborns and small infants in particular.1
Conclusion
The study found that newer techniques such as BMI were more precise in identifying particulates, which raises questions about the applicability of current USP guidelines to pediatric pharmacy practice. While these findings signify steps forward in improving clinical results and reducing IV access requirements for pediatric patients, further chemical compatibility testing is still needed.1
Related
- Learn more how light osbscuration can potentially dangerously undercount particles
- See how we do analysis for USP 788 compliance
- Watch our play by play on how to validate Aura for USP 788
References
1. Ross, Emma L., et al. “Physical Compatibility of Y-site Pediatric Drug Administration: A Call for Question of US Pharmacopeia Standards?” The Journal of Pediatric Pharmacology and Therapeutics, vol. 28, no. 1, 2023, pp. 84-92, https://doi.org/10.5863/1551-6776-28.1.84.
DISCOVER THE AURA FAMILY
Read More ON this topic









