Why USP 788 Matters: Ensuring Patient Safety through Particulate Matter Testing in Injections
November 8, 2023
Why USP 788 Matters: Ensuring Patient Safety through Particulate Matter Testing in Injections
In the world of pharmaceuticals, strict regulations are crucial for protecting patients and ensuring product efficacy. One significant regulation is the United States Pharmacopeia Chapter 788 (USP 788), which provides guidelines for testing particulate matter in injections.
But why is this important? Particulate matter, often too small to see with the naked eye, can pose significant risks – limiting both a product’s shelf life and compromising patient safety.
If these subvisible particles (1-100 µm) enter a patient’s circulatory system through an injection, it can lead to inflammation or even blockages in blood vessels. That’s why injectable drugs must meet stringent requirements to minimize this risk.
The source of these subvisible particles can vary — from protein-active pharmaceutical ingredients (API), degraded excipients, and/or particles from the container system to contaminants introduced during manufacturing, packaging, or other external processes. This makes the detection and quantification of particulate matter a critical task, precisely what USP 788 aims to achieve.
Two Methods for USP 788 Particulate Matter in Injections
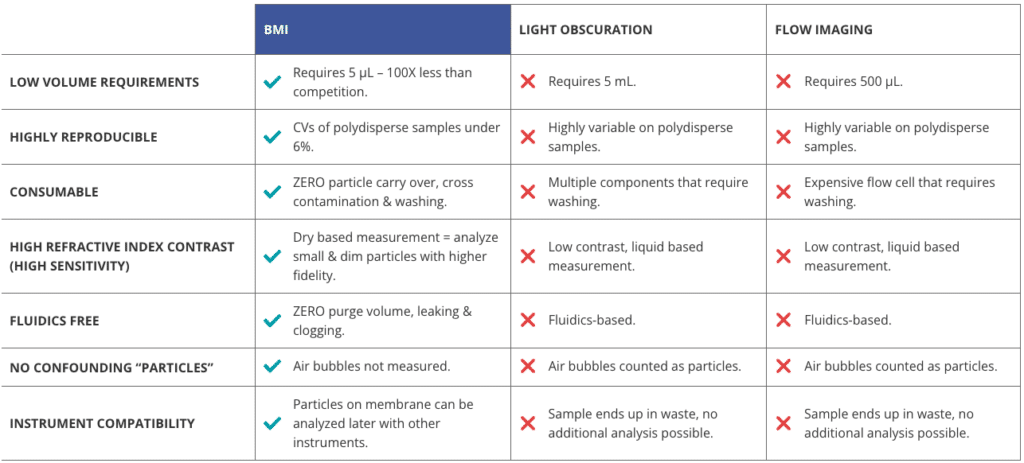
USP 788 specifies two methods for determining particulate matter: Method 1 – Light Obscuration Particle Count Test and Method 2 – Microscopic Particle Count Test.1
The light obscuration (LO) particle counter is an instrument that uses a light source and detector to determine the size and concentration of particles suspended in a specific sample. The instrument measures the degree of light blockage caused by individual particles by passing the sample through a narrow aperture.

Light obscuration is a commonly used method for quantifying particles in the ≥ 10 µm and ≥ 25 µm size ranges, although it can also measure particles as small as 2 µm. However, this technique has its limitations. It has difficulties accurately counting particles in high-viscosity formulations and faces challenges in assessing particle morphological information.2 Often, transparent and non-spherical particles do not adequately obscure light and are, therefore, frequently undercounted by this method.2
The Need for Better Particulate Matter Testing and Analysis Technology
Given the critical nature of developing therapeutics, understanding and controlling physical stability during all stages of the manufacturing process is vital. Manufacturers cannot rely solely on minimum standards when releasing a drug — particles must be fully characterized at every stage.
To achieve this, researchers require the most effective tools for monitoring particle levels to demonstrate control and safety. While USP 788 recommends using light obscuration techniques and a microscopic particle count test for particulate analysis (USP 788 Method 2), reliance on light obscuration alone falls short in providing a holistic picture, especially for high viscosity formulations or extrinsic particle contamination.
This calls for a solution that can deliver a precise and accurate technique for characterizing subvisible particles as well as contaminants.
Backgrounded Membrane Imaging: A Game-Changer for Generating USP 788 Compliant Data
Halo Labs utilizes Backgrounded Membrane Imaging (BMI), a high-contrast imaging technique, to generate accurate USP 788-compliant subvisible particle (SVP) data.
This technique has been developed on Aura®, the first system that allows particles to be tested both early in development and later during quality control. With Aura, you can determine if a particle is the result of the drug itself, an ingredient in the liquid crashing out of solution, an external contaminant, or even a cell.
Backgrounded Membrane Imaging reinvents membrane imaging with modern robotics, image processing, and novel optics in a 96-well filter plate format that works just like a plate reader. It’s also insensitive to solution refractive index, enabling reliable measurement of translucent protein aggregates.
Leveraging Aura for Efficient Particle Counting in Accordance with USP 788 Method 2
Following the USP 788 Method 2 guidelines, we’ve developed an efficient and accurate particle counting protocol for lot release testing using Aura.
With our high-throughput method, pre-washing, backgrounding the membrane, filtering the sample, and washing the membrane again after filtration are required steps to successfully execute an assay in a fraction of the time compared to traditional methods. Efficiency is further heightened when combined with robotic handlers due to our plate’s compatibility with robots to give you even faster results.
The procedures for washing membranes recommended by USP 788 have a big benefit: they increase the reliability of particle counts. After the sample has been applied to the membrane, any soluble material, such as proteins and salt crystals, that may cloud the results is washed away with ultra-pure water. And because Aura can perform multiple measurements, all assay measurements can be more easily validated, adding another level of confidence in their accuracy.
Conclusion
Aura systems generate accurate USP 788-compliant subvisible particle data, perfectly complementing light obscuration so drug producers have complete coverage of all particles of interest.
By moving on from antiquated techniques, you’ll have the power to make smarter decisions faster, ensuring critical therapeutics are developed safer and quicker, in order to help patients in need.
Related
- Light Obscuration May Not Be Enough to Detect SVPs in Your Drug Product
- Lot release testing and QC with Aura
- Check out our “How-To” count USP particle standards with Aura and Particle Vue
References
DISCOVER THE AURA FAMILY
Read More ON this topic