Overcome One of Cell Therapy's Biggest Challenges
If you need to make sure that your cell therapy is both safe and effective, and meets USP 1046 standards, traditional flow cytometry methods simply won't cut it.
Light obscuration (LO) and flow imaging (FI) only provide ~50-60% accuracy when cells clump or are out of focus1—not exactly a huge improvement. Plus, ancillary components throughout production must be tracked as part of the final product or removed if not applicable.
The good news? All these challenges can become a thing of the past with Aura+ and Aura CL—fluidic-free particle analyzers that utilize Backgrounded Membrane Imaging (BMI) and Fluorescent Membrane microscopy (FMM) so you can get precise measurements for cell therapy quality control. These advanced technologies significantly enhance the robustness and reliability of the therapy manufacturing process by providing accurate and comprehensive particulate analysis.
- Wang X, Rivière I., (2016) Clinical Manufacturing of CAR T cells: Foundations of a Promising Therapy. Molecular Therapy Oncolytics. 3:16015. doi: 10.1038/mto.2016.15
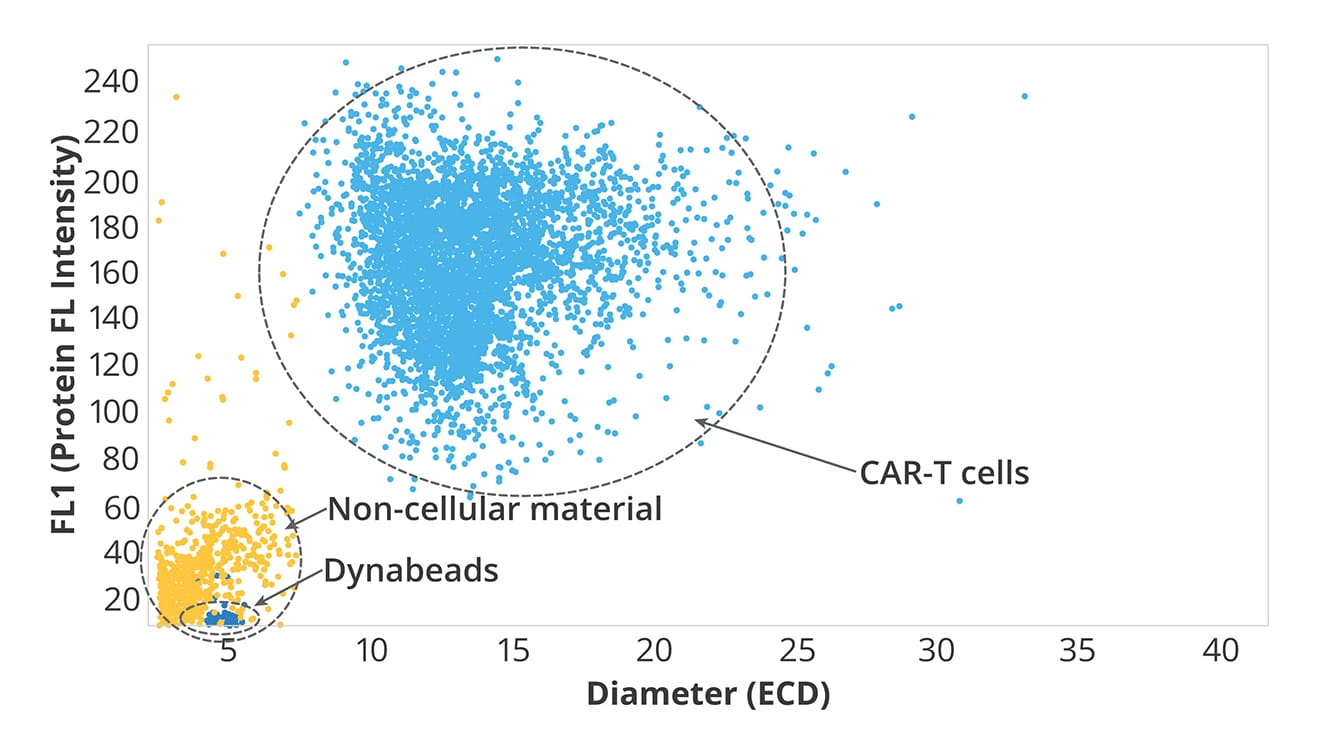
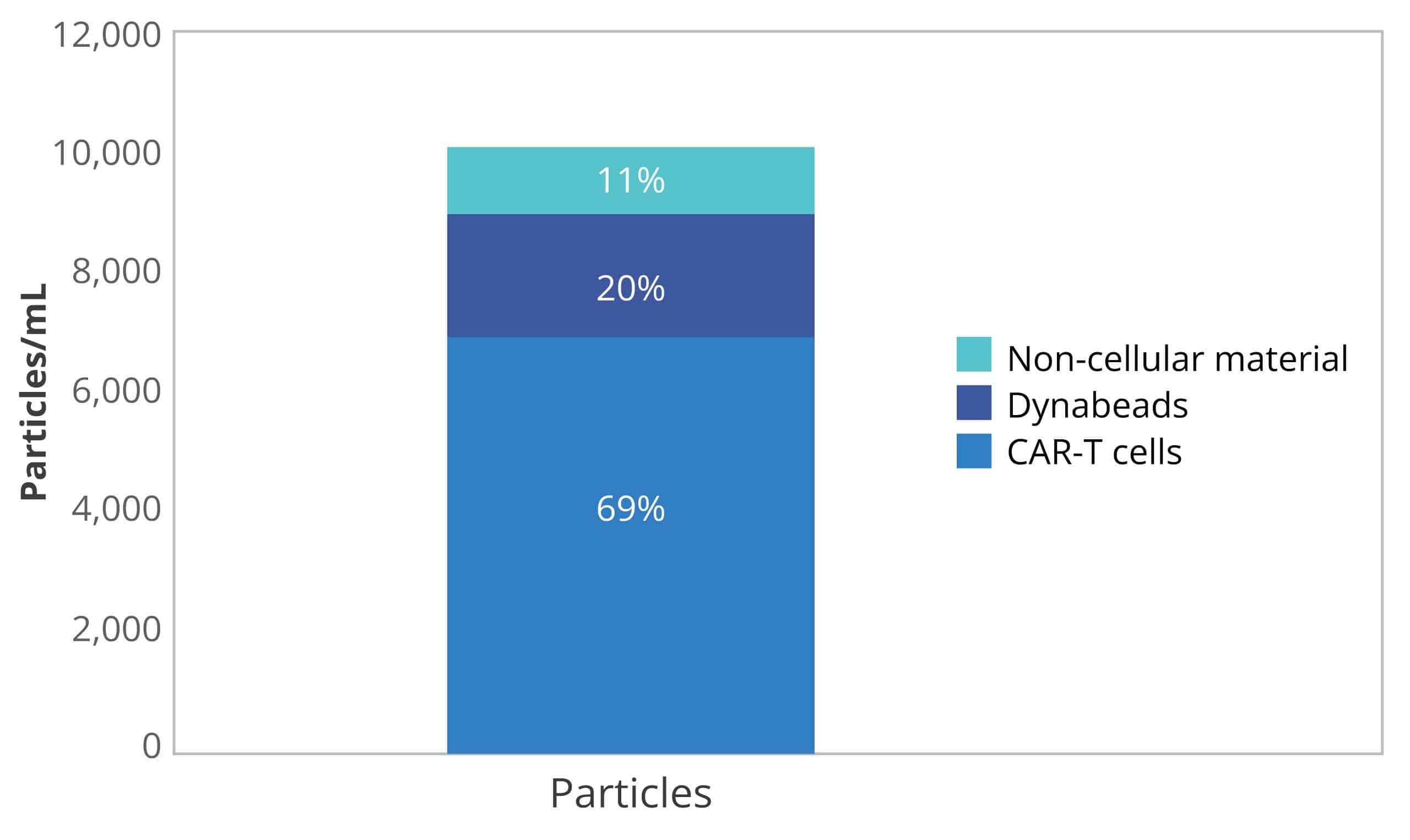
Why Use Aura to Assess Cell Therapy Product Purity?
- Achieve accurate, fluidics-free subvisible particle analysis
- Definitively ID cell and non-cell subvisible particles
- Best in class Dynabeads™️ identification and quantitation
- Extrinsic particle identification and characterization
- Obtain detailed information on particles that other methods can’t deliver, including size, morphology, count, and distribution
- Rapid analysis time of about 1 minute per sample
- Benefit from a wide working range: measure particles from 1 μm to 5 mm with high reproducibility
- Image particles without the interference of buffer or matrix for higher sensitivity
- High-resolution particle images
- USP 788 Method 2 and USP 1046 compliant
- Maintain compliance with the option for 21 CFR Part 11 software
*Dynabeads refers to the magnetic beads produced by Thermo Fisher Scientific, Inc. Halo Labs is not affiliated with Thermo Fisher Scientific, Inc., and references to Dynabeads or any other third-party trademark do not imply sponsorship, endorsement, or approval.
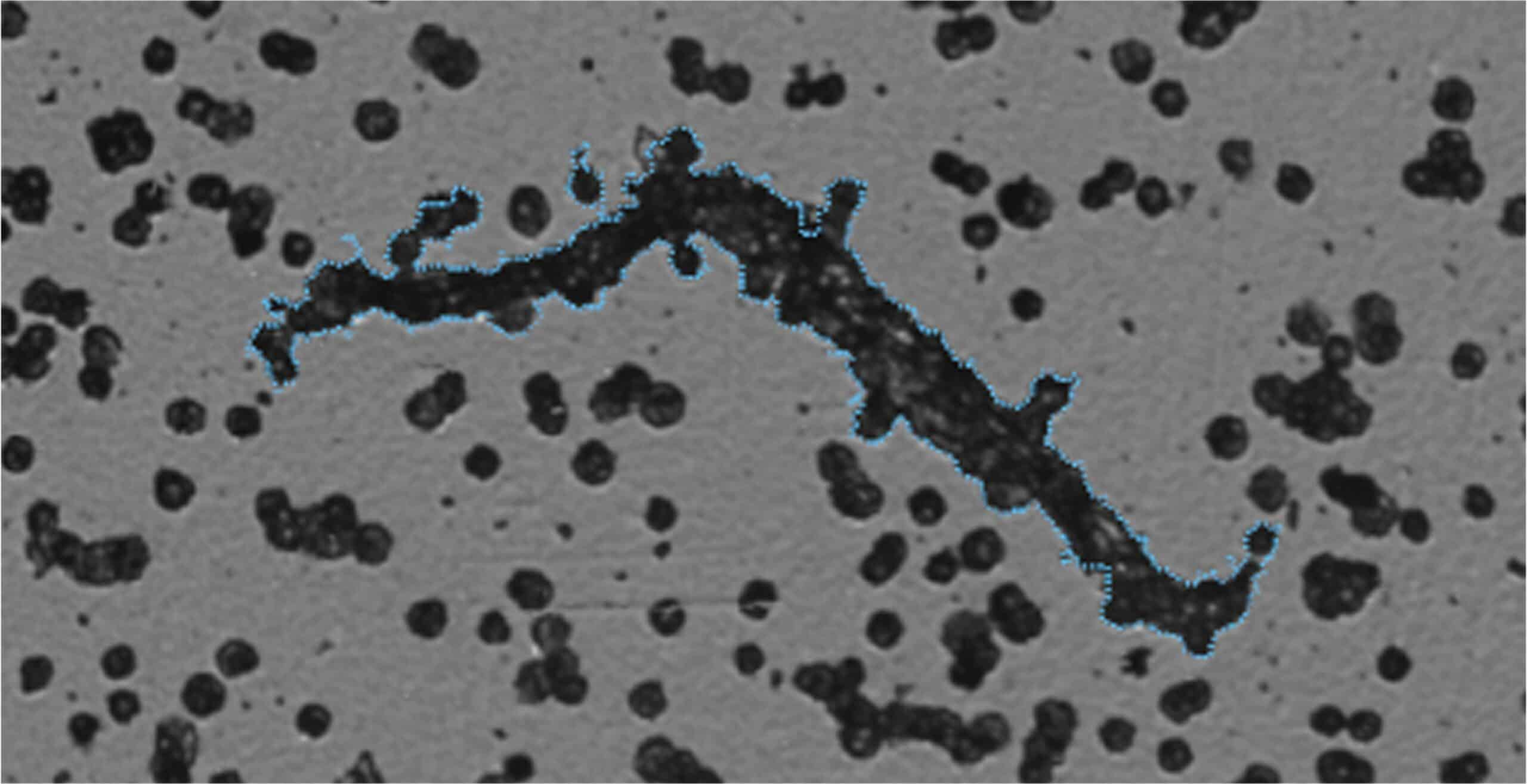
Zoom in on Particles of Interest with High Magnification Imaging
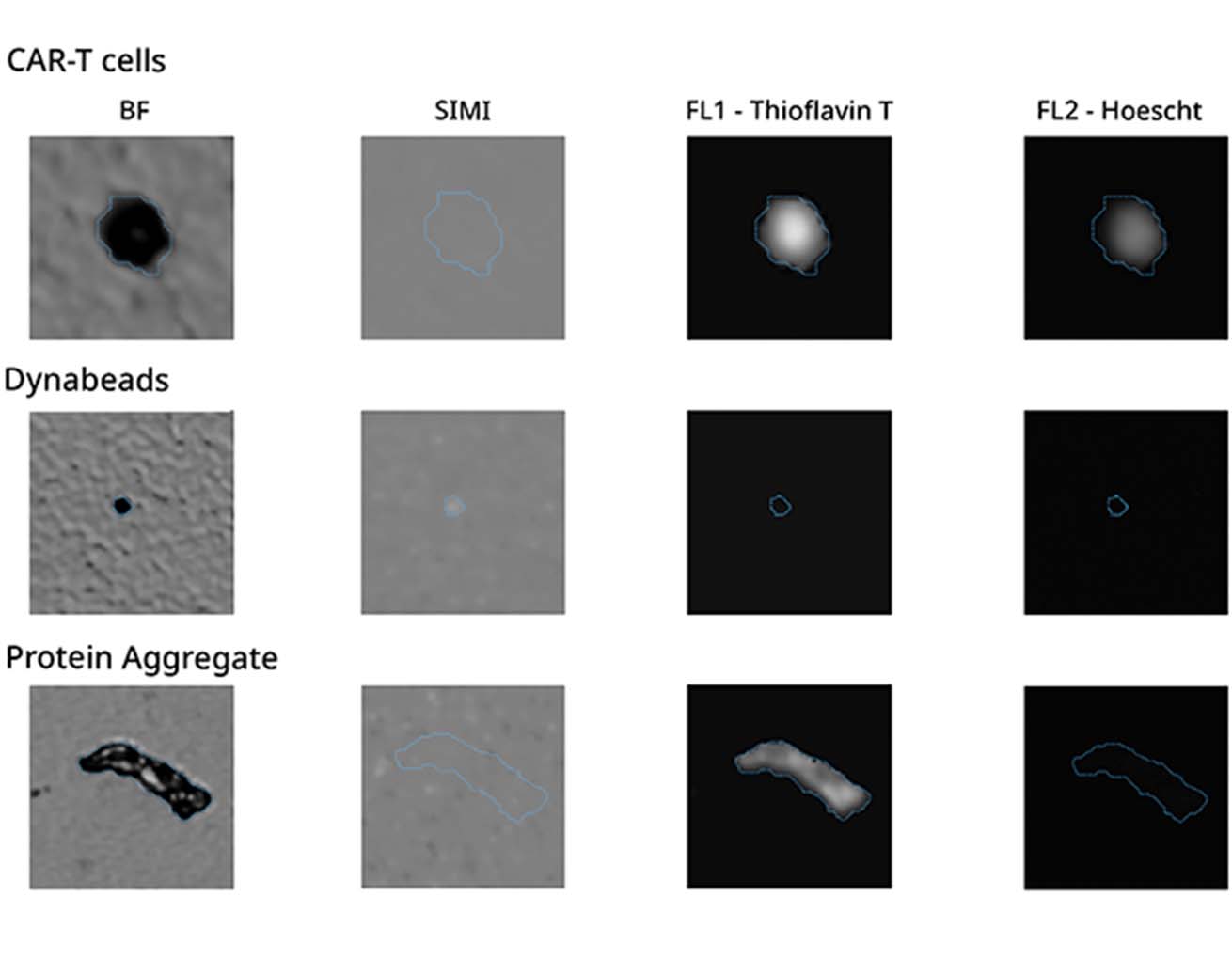
It can be hard to tell the difference between particles with similar shapes, especially if they are not labeled in your sample. Now, you can use a combination of brightfield imaging and FMM to keep an eye on extrinsic process contaminants like fibers and make sure that their concentration won't affect the safety or effectiveness of the product.
Need an even closer look? Because it uses a 20x objective, Aura+ provides additional morphological data about particles in your sample, so you can more clearly determine product purity during cell therapy quality control.
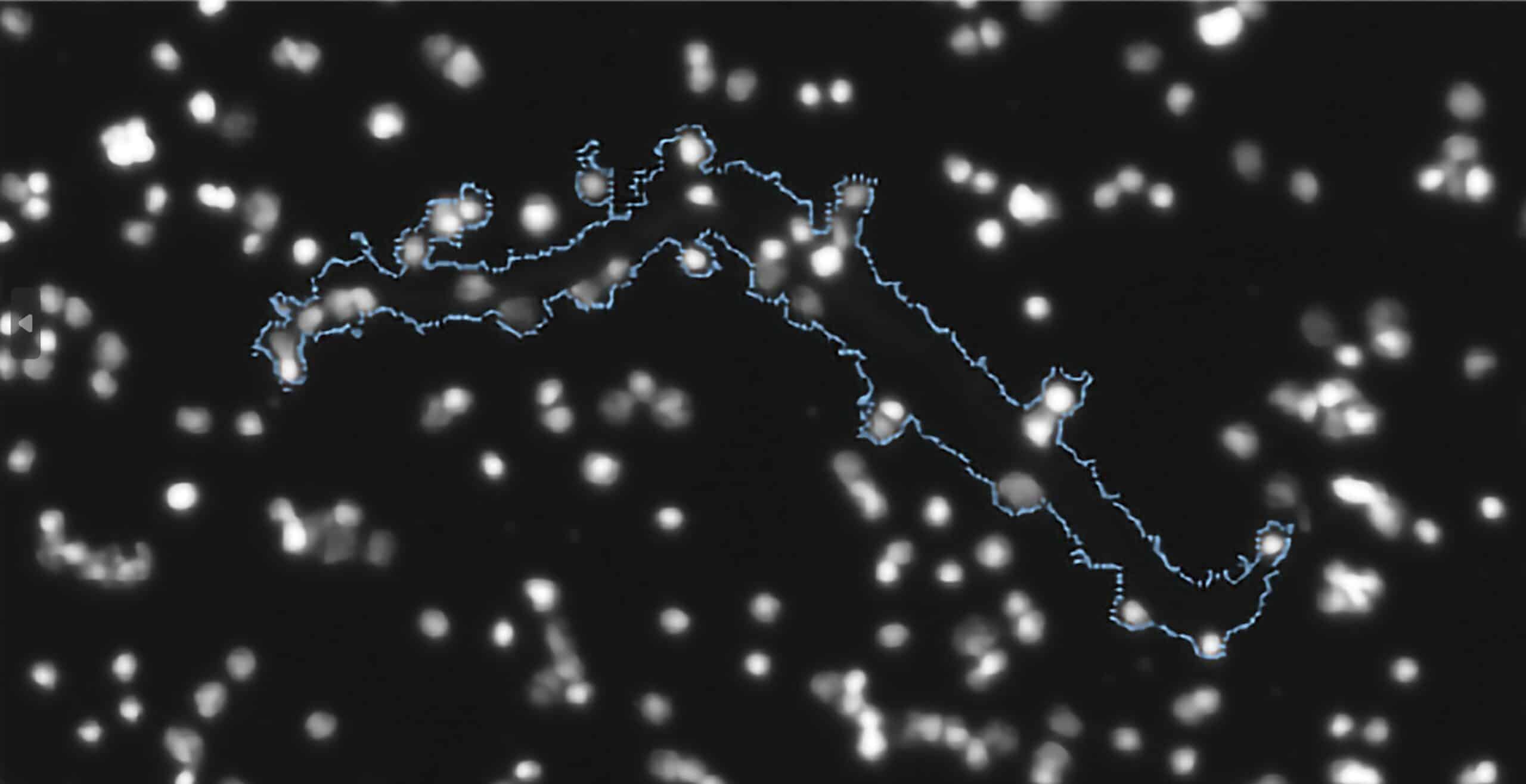
Make FMM Your Go-To Tool for Distinguishing Subvisible Particles
Both Aura+ and Aura CL use fluorescence signatures via FMM instead of morphology for identification, making cell therapy quality control simpler than ever.
Add to that Side Illumination Membrane Imaging (SIMI), which picks up non-biological and inorganic particles hidden in samples, allowing you to quickly distinguish even more types of subvisible particles without complex machine learning algorithms.
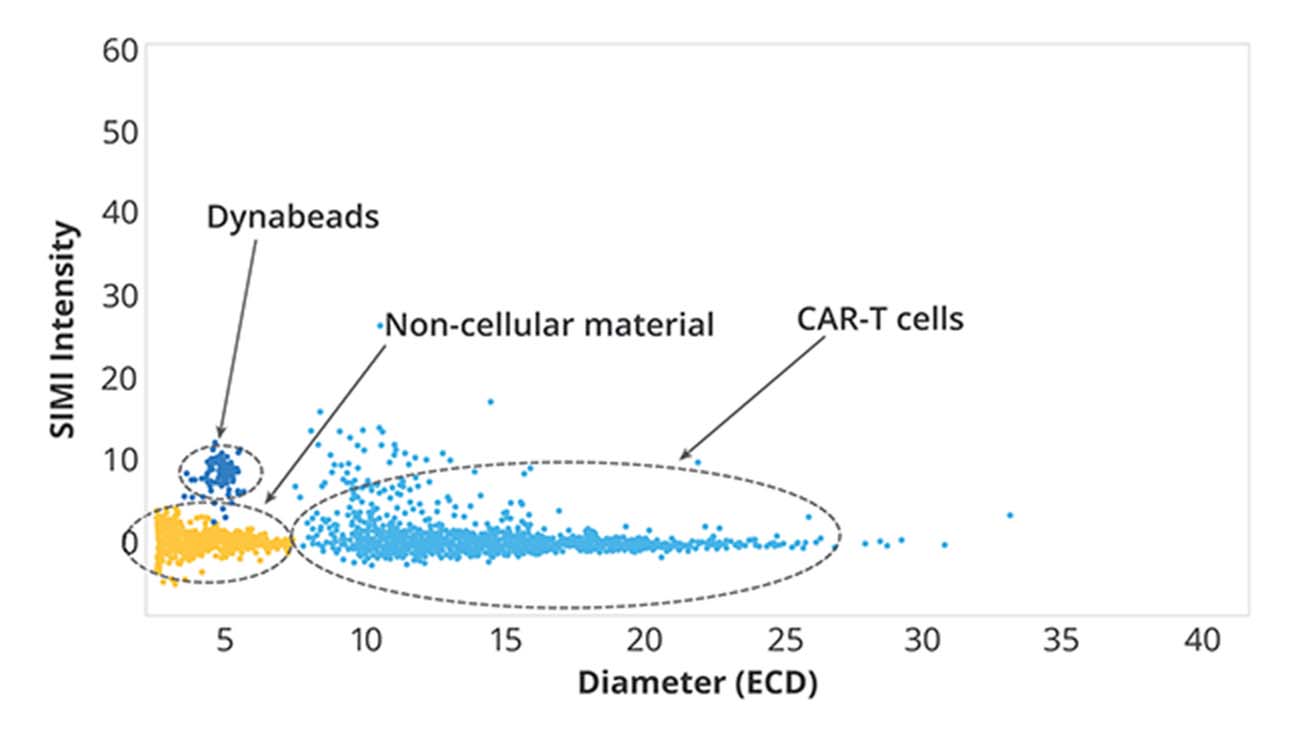
Detect Dynabeads with SIMI for Safer Drugs
Flow cytometry might seem like an obvious choice for cell-based therapy quality control, but it often falls short. Sample prep can be a challenge, and clogging caused by Dynabeads and cells renders it obsolete.
This is where SIMI provides a much-needed solution. Using side-illumination, it can accurately detect non-biological objects at the membrane level, such as Dynabeads, which must be identified and removed before use in cell therapies.
To make this process more efficient, Aura CL and Aura+ offer an automated way to count Dynabeads in highly cell concentrated solutions, making it possible to deliver reliable and error-free results with greater accuracy and throughput
Featured products
Frequently Asked Questions
The quality control of CAR-T cell therapy involves a series of rigorous tests and evaluations to ensure the safety, purity, potency, and consistency of the final product. This includes assessing the identity and purity of the starting cell population, verifying the genetic modification of cells, monitoring cell viability and potency, and testing for microbial and endotoxin contamination. Additionally, comprehensive release testing is performed to confirm that the final product meets predefined quality specifications before administration to patients.
In the context of cell and gene therapy, QC stands for Quality Control. QC in cell and gene therapy refers to the set of procedures and processes implemented to ensure that the products meet predefined quality standards and specifications. These standards encompass various aspects including safety, purity, potency, and consistency of the therapeutic products. QC measures involve rigorous testing, analysis, and monitoring throughout the manufacturing process to identify and mitigate any deviations or impurities that could affect the efficacy or safety of the final therapy. QC is crucial for ensuring the reliability and effectiveness of cell and gene therapies before they are administered to patients.
In addition to these measures, QC also requires thorough characterization of various quality attributes of the therapeutic products. Quality attributes such as identity, strength, purity, and stability must be monitored to ensure consistency and predictability of therapy outcomes. During clinical trials, careful attention is paid to the selection of cell types used in gene therapy, as different cell types may respond differently to treatment protocols and impact the therapeutic efficacy. Ensuring that these quality attributes are met and maintained is essential for the successful translation of these therapies from clinical trials to real-world applications.