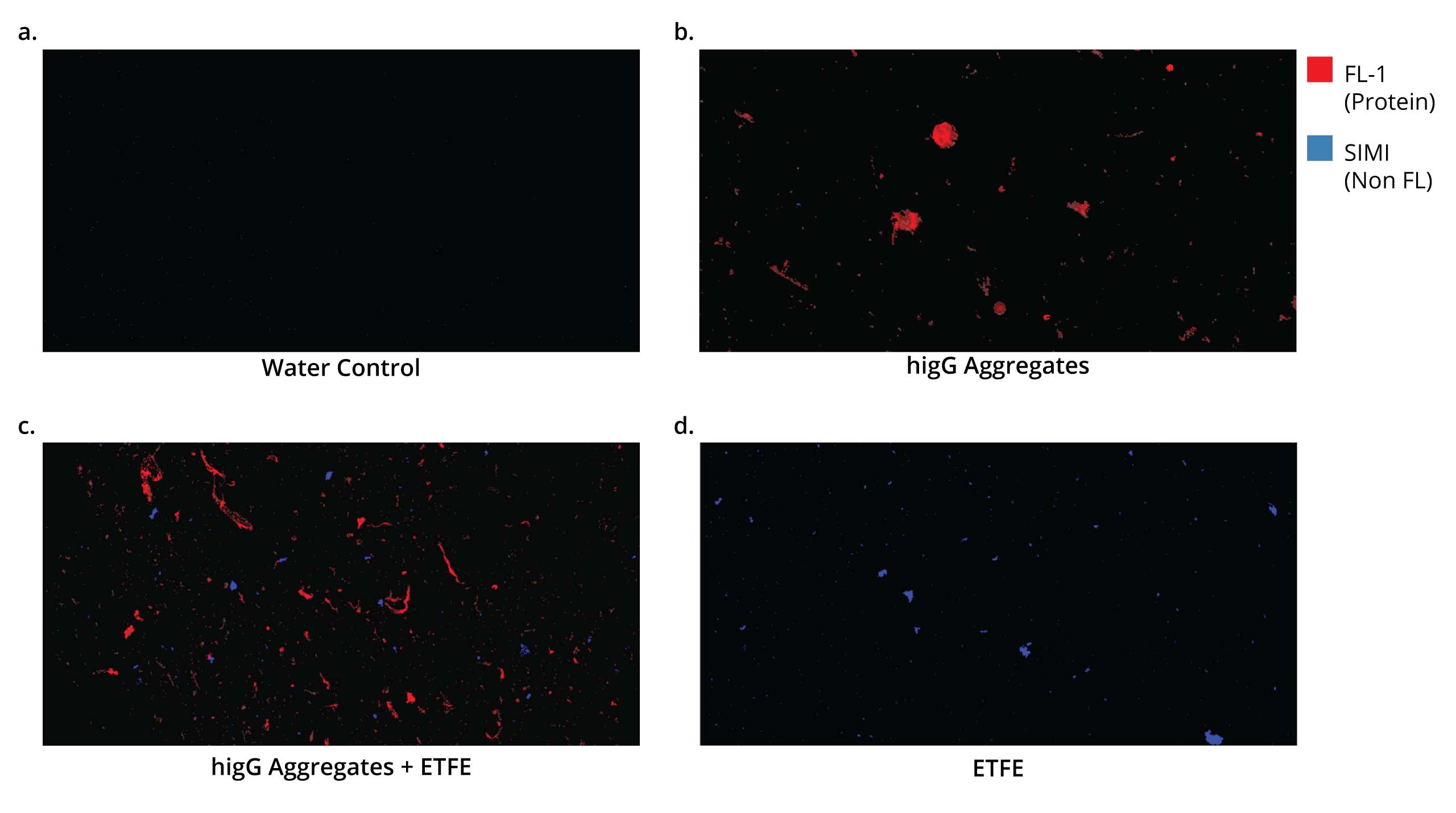
Protein Quantification Upscaled
Precise protein quantification is essential for research on biopharmaceuticals. Quantification dictates the effective and safe dose of protein therapies and determines the quality of a therapy by measuring protein vs non-proteins. In addition, measuring antibody titers can help in diagnosing diseases as well as fine-tuning their use in therapies. Aura+ and Aura PTx can quickly quantify protein aggregates in a sample, and identify proteins from non-proteins requiring very little sample.
Why Use Aura for Protein Quantification Analysis?
The Aura family of instruments can overcome the limitations experienced with more traditional protein quantification methods. These five methods all have disadvantages:
- Bradford/Coomasie Blue Test—where negative Coomasie blue stains bind positively charged proteins. While fast and simple, it cannot detect protein aggregates as aggregates often have altered structures and may not bind the dye in the same way as soluble proteins and is not compatible with SDS or Triton X-100 detergents
- Lowry Test—Formation of Cu-N complexes, then tyrosine and tryptophan react with Folin-Ciocalteau reagent, producing a blue-green color. The test is sensitive and accurate but is not compatible with common reagents such as Tris, EDTA, DDT, 2-mercaptoethanol, and carbohydrates
- BCA—Bicinchoninic acid test where absorbance is proportional to protein concentration. Sensitive and recommended for samples containing detergents/denaturing agents, but samples containing ammonia, EDTA, reducing sugars, or lipids may interfere with the assay, and colors are not stable
- Biuret—based on the formation of a colored complex between Cu2+ and NH groups of peptide bonds. While fast, it is insensitive and requires 2 to 4 mg of proteins for testing
- UV absorption—measuring absorption of tryptophan and tyrosine at 280 nm, estimates the amount of protein quickly. However, it is not compatible with protein extractions that use detergents/denaturing agents and is not specific to proteins
- Clearly, better methods of protein quantification are needed when looking for aggregates or insoluble protein - Aura+ and Aura PTx instruments are more accurate, rapid, sensitive, and versatile, meeting the increased demands of today’s research and development of biological therapies
Optimized Biopharmaceutical Lot Release
Protein quantification of aggregates is important for successful lot release, and the right testing technologies are needed to accurately determine that your sample therapy meets the required specifications and is compliant with USP 788 and other regulations under 21 C.F.R. Part 610.
During the lot release step, drug substances and finished products are analyzed for any impurities or degraded materials that can impact the drug’s actions, as well as the structural and functional integrity of the substance itself. These tests are provided to the FDA in advance of drug approval and provide real-time monitoring of drug substances.
Aura+ and Aura PTx enhance the lot release step due to their more accurate and reliable quantification of protein in a sample, and better identification of aggregates and contaminants that can impact protein quantity and purity. Having a better grasp of what is in your sample means reduced risks of product holds and delays from changing processing and manufacturing procedures.
Improve Your Detection
Accurate protein quantification is essential to developing an effective, safe biotherapeutic that meets USP guidelines and federal regulations. To arrive at a reliable protein count, we need to go beyond what traditional techniques offer, probing into the “subvisible” range that reveals details that are otherwise hidden. With Aura systems, you get detailed information on particles, protein and non-protein, that other methods can’t deliver, with analysis time of about 1 minute per sample and using as little as 5 µL of sample.
Featured products
Frequently Asked Questions
Protein quantification is the measurement of total concentration of protein in a sample. Aura+ and Aura PTx systems quantify aggregated proteins with precision and sensitivity: Backgrounded Membrane Imaging (BMI), which delivers count, size and morphology, and Fluorescence Membrane Microscopy (FMM), which differentiates and identifies protein, cellular, and other aggregates and extrinsic particles.
Traditional quantification methods include: the Bradford/Coomasie Blue Test, Lowry Test, BCA, Biuret, and UV absorption. All of these have disadvantages, due to inaccuracies, incompatibility with common reagents, or the need for large sample sizes. Aura+ and Aura PTx systems use different technologies which have yielded more accurate protein counts by using far less samples.
Proteins can be quantified using absorbance (at 280 nm) or by using colorimetric (staining) or fluorescence methods. The Halo Labs Aura system adds another level of precision with BMI and FMM, which compares membrane images before and after samples are filtered (BMI) and uses fluorescent labels such as Thioflavin T (FMM) to identify and categorize proteins and particles in your sample. A more advanced FMM approach would be utilizing immunoassays for specific protein quantification.
At Halo Labs, we have shown that Aura instruments provide more accurate protein aggregation quantification data compared to other traditional methods.