Overcoming the Challenge of Polysorbate Degradation
Polysorbates (e.g., polysorbate 80) and Tween™ are the go-to formulation excipients for biologic development due to their ability to minimize protein aggregation and improve product stability.
The downside: free fatty acid (FFA) particles form when polysorbate is stored for long periods at low temperatures, which can have devastating consequences. Traditional methods of analysis like light obscuration (LO) and flow imaging (FI) cannot identify degradation sources.
To get more detailed data, you need a high-throughput solution, which is easier said than done given polysorbate’s complicated chemistry and low concentrations.
At Halo Labs, our pioneering Aura technology allows us to do something that no other company can - provide high-precision fluorescence labeling of free fatty acid for biologics.
Why Use Aura to Monitor Polysorbate Degradation?
Because you can achieve so much more insight with much less material:
- Complete biologic particle characterization for stability
- Definitively ID free fatty acids, the degradation product of polysorbate
- Gather comprehensive data and insight into particles – size, morphology, counts, distribution
- Sample volumes that meet your requirements – accurate analysis with 5 µL – 10 mL of sample
- High-throughput 96-well format for testing lots of conditions
- Rapid analysis time of about 1 minute per sample
- High-resolution particle images
- Automated data analysis with Particle Vue software
- 21 CFR Part 11 compliant software upgrade available
BMI + FMM Technology Set Aura Apart
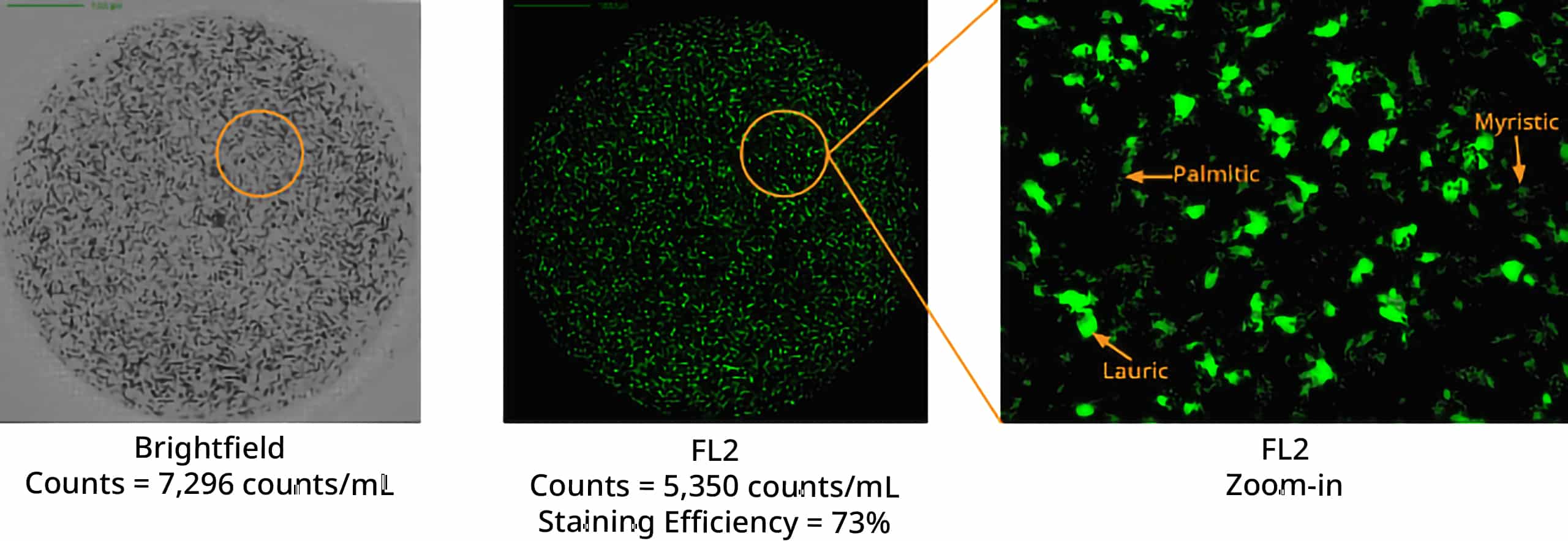
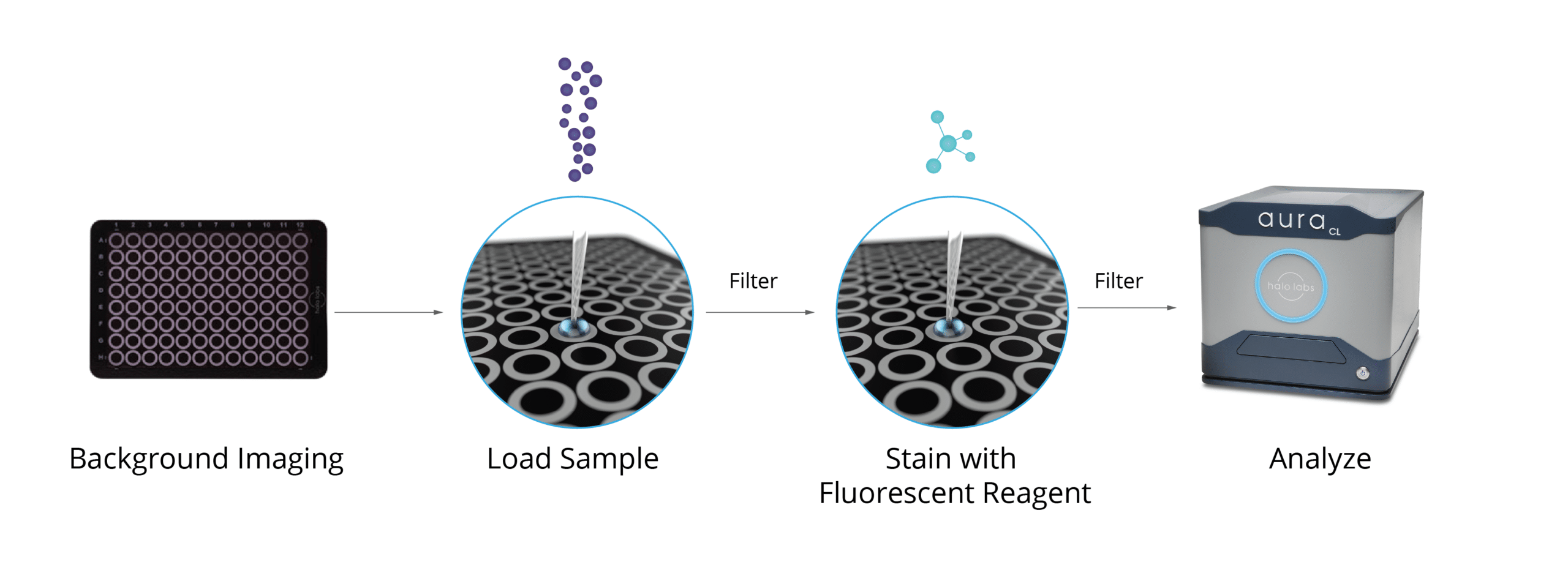
Aura systems utilize two cutting-edge methods, Backgrounded Membrane Imaging (BMI) and Fluorescence Membrane Microscopy (FMM), to accurately detect free fatty acid particles in a sample. With these powerful imaging techniques, it is now possible to measure free fatty acids with an accuracy that has never been seen before.
To further amplify measurement results, fluorescent stains like Thioflavin T (ThT), a sensitive fluorescent dye, can be used to identify and analyze subvisible protein formulation excipients. Aura systems can be employed in excipient testing to ensure the compatibility and stability of the formulation components, as well as to determine the fatty acid composition, ensuring the overall quality control and safety of the final product.
Get Accurate, Quantitative Results in Hours
With Aura's polysorbate degradation assay, you won't have to spend days combing through irrelevant data in search of a needle in a haystack — polysorbate concentrations are also much lower (<<0.5%) than biologic concentrations (>100 – 200 mg/mL).
This method can be used with as little as 5 µL to as much as 10 mL of sample, and it can be used to test as many as 96 samples at once for the presence of free fatty acid particles, which form when polysorbates degrade.
Identify Free Fatty Acid Particles
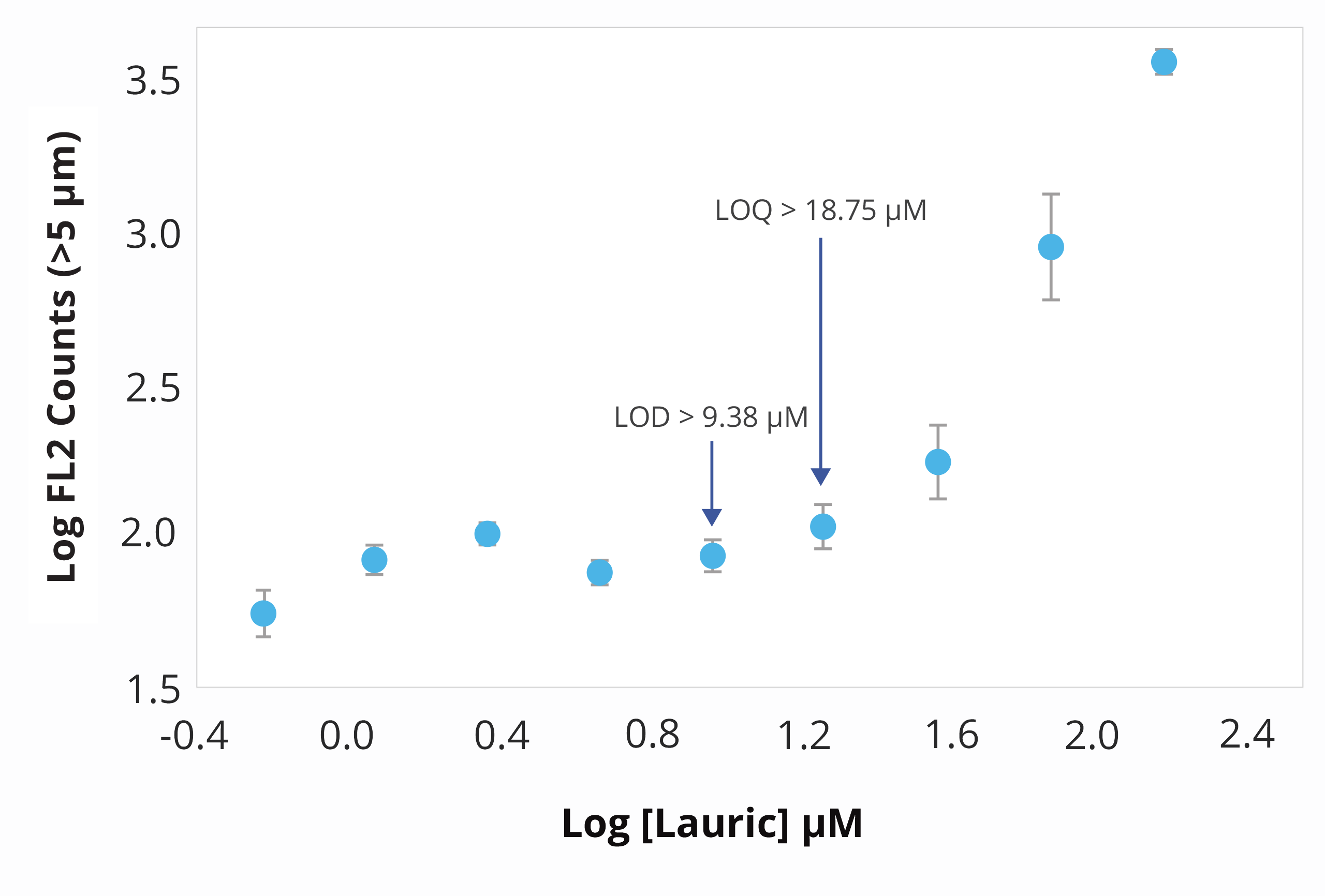
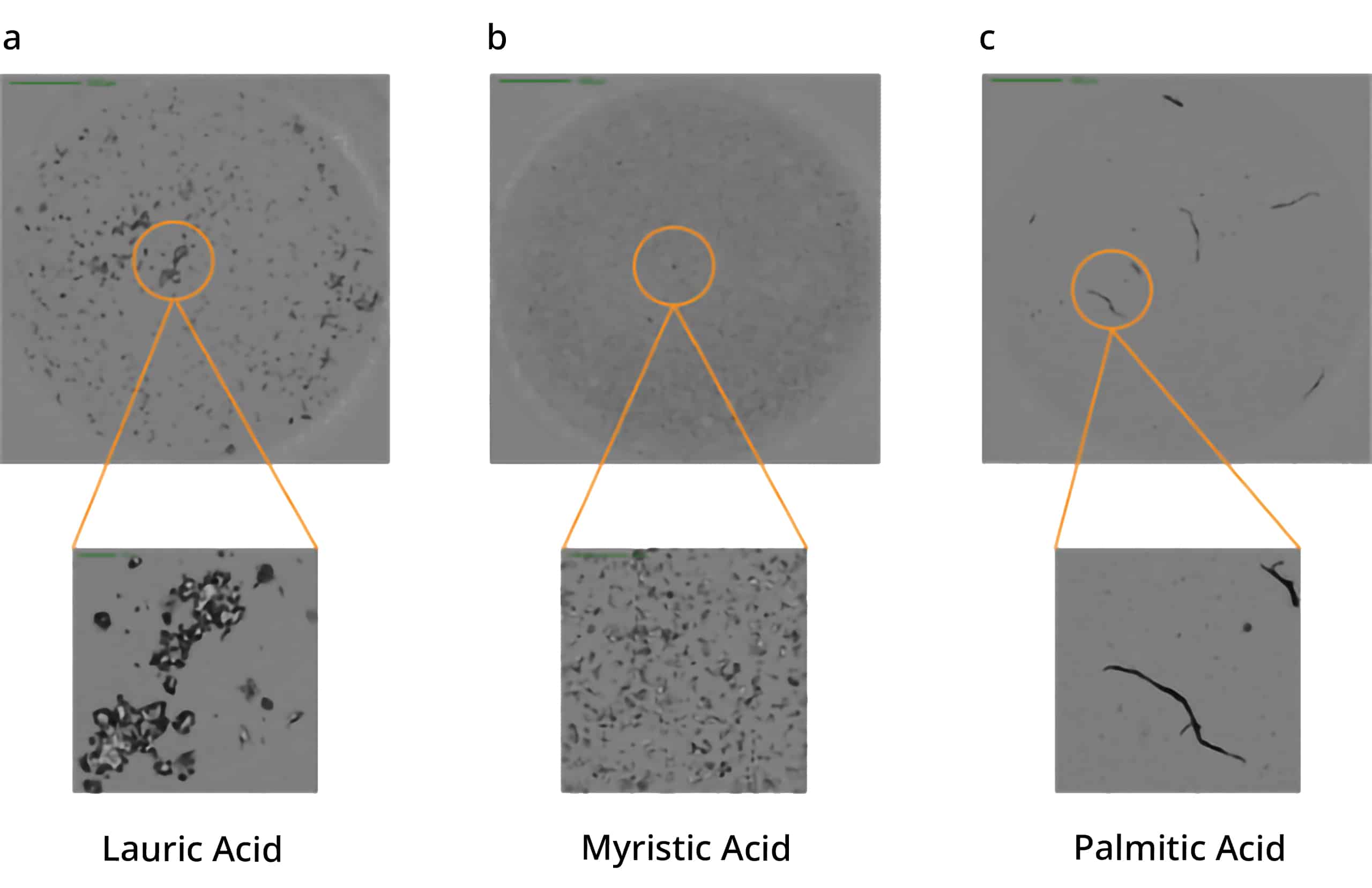
Polysorbate degradation can break down into three distinct fatty acids: lauric, myristic, and palmitic acid, leading to the formation of particles with distinct morphological characteristics.
Using Aura's advanced labeling and imaging, you can differentiate particles down to the subvisible level and detect unique morphological characteristics. To identify free fatty acids specifically and accurately, BODIPY™ FL C16 offers a powerful stain with high affinity that will help you make sense of your sample.
This offers an opportunity for detailed analysis of free fatty acid particles that can then be confirmed with FMM using labeled fluorescence.
Featured products
Frequently Asked Questions
Fatty acid analysis is a biochemical technique used to determine the composition and concentration of fatty acids present in biological samples or food products. Fatty acids play essential roles in metabolism, cellular structure, and signaling pathways, and their composition can influence various physiological processes and health outcomes. Fatty acid analysis helps researchers and clinicians understand the lipid profile of biological samples, assess nutritional quality, diagnose metabolic disorders, and investigate disease mechanisms. By quantifying fatty acid levels and profiles, researchers can gain insights into lipid metabolism, dietary intake, and the impact of fatty acids on health and disease, facilitating personalized nutrition interventions and therapeutic strategies.
In the case of biologics developers, free fatty acid analysis provides insight into the stability and effectiveness of polysorbate, which is a common excipient for biologics formulations.
Polysorbate 80, also known as Tween 80, is a commonly used surfactant and emulsifier in pharmaceutical formulations and food products. However, polysorbate 80 has been associated with several concerns, including hypersensitivity reactions, anaphylaxis, and adverse effects on product stability. In some cases, polysorbate 80 can contribute to protein aggregation or degradation, leading to reduced product efficacy or immunogenicity. Additionally, polysorbate 80 may interact with other excipients or drug substances, affecting formulation compatibility and stability. Due to these potential issues, pharmaceutical manufacturers must carefully evaluate the use of polysorbate 80 in formulations, consider alternative excipients or formulation strategies, and conduct appropriate stability and compatibility studies to ensure product safety and efficacy.
For more information, check out our blog post on polysorbate and its impact in IgG stability.
Excipients are inert substances added to pharmaceutical formulations to impart specific properties, aid in drug delivery, or improve product stability, appearance, or palatability. Common types of excipients used in formulation include:
- Fillers and diluents: These add bulk to solid dosage forms, facilitate uniform tablet compression, and improve flow properties. Examples include lactose, microcrystalline cellulose, and mannitol.
- Binders and adhesives: These promote tablet cohesion and integrity by binding powders together during compression. Examples include starches, cellulose derivatives, and polyvinylpyrrolidone (PVP).
- Disintegrants: These facilitate tablet disintegration and drug dissolution in the gastrointestinal tract, enhancing drug absorption. Examples include croscarmellose sodium, crospovidone, and sodium starch glycolate.
- Lubricants and glidants: These reduce friction during tablet compression, improve powder flow properties, and prevent tablet sticking. Examples include magnesium stearate, stearic acid, and colloidal silicon dioxide.
- Surfactants and emulsifiers: These stabilize emulsions, enhance solubility, and improve drug bioavailability. Examples include polysorbates (e.g., polysorbate 80), lecithin, and sodium lauryl sulfate.
- Preservatives and antioxidants: These prevent microbial growth, oxidation, and degradation of active pharmaceutical ingredients (APIs) or formulations. Examples include benzalkonium chloride, sodium benzoate, and ascorbic acid.
- pH modifiers and buffers: These maintain the desired pH of formulations, improve stability, and enhance drug solubility. Examples include citric acid, sodium bicarbonate, and phosphate buffers.
- Colorants, flavors, and sweeteners: These improve the appearance, taste, and palatability of pharmaceutical formulations, enhancing patient acceptability. Examples include FD&C dyes, artificial flavors, and sucrose.