Stop Invisible Aggregates Before They Destroy Your Gene Therapy
The complexity of lentiviral vectors (LVVs) presents many challenges when it comes to gene therapy manufacturing.
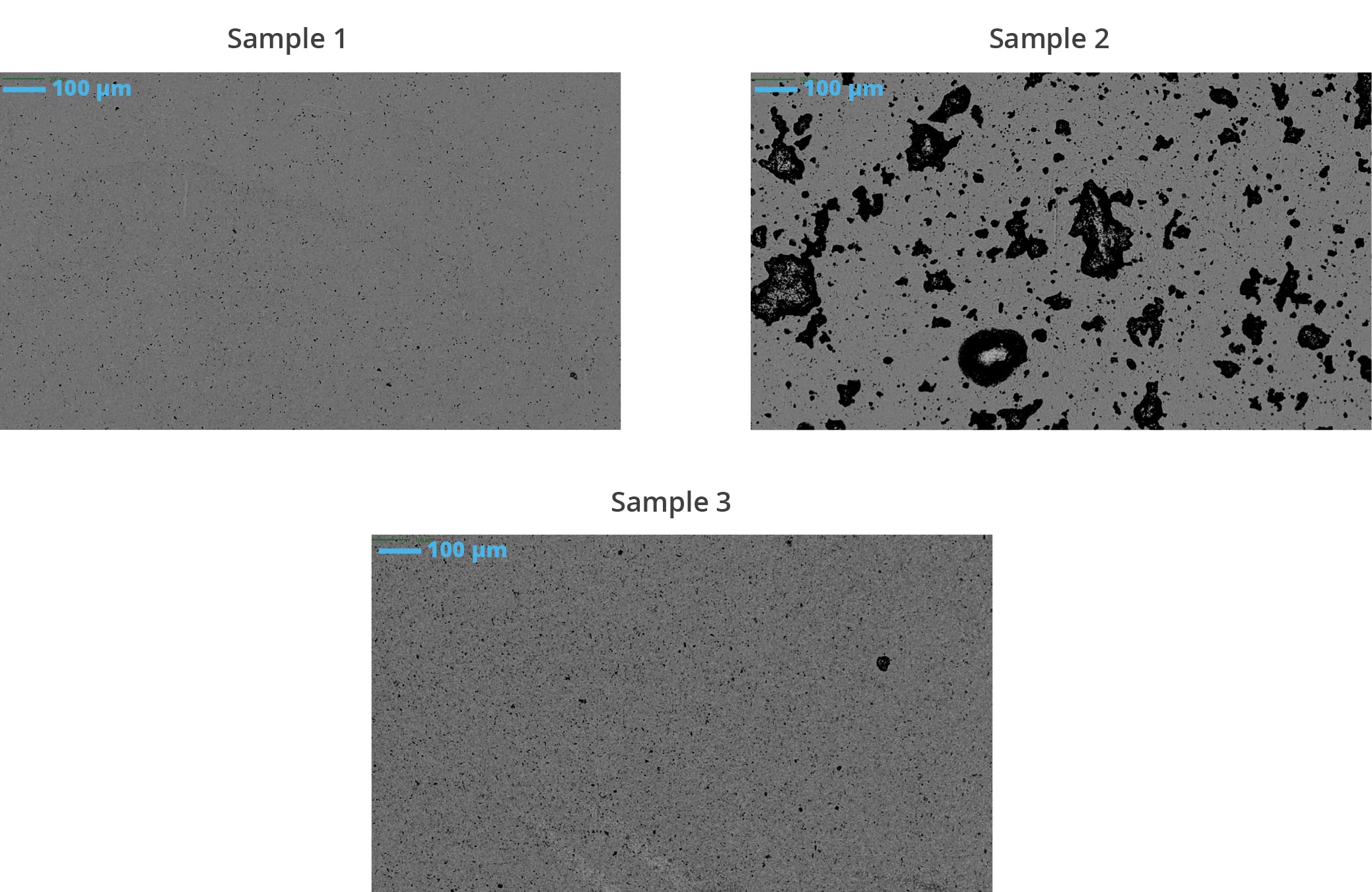
Compared to protein biologics, LVVs can be more difficult and expensive to manufacture. The scarcity of LVV material also makes scale-up difficult and adds additional complexity when qualifying subvisible particles, the most indicative critical quality attribute for lentivirus stability.
What if there was a way to overcome these challenges?
With Aura GT, there is.
Why Use Aura to Measure Lentivirus Stability?
Because you can achieve so much more insight with much less material:
- Achieve more accurate results with minimal amounts of sample (as little as 5 μL per test)
- Identify SYBR™-labeled nucleic aggregates from unstable viral vectors
- Detect and quantitate particles not measured by DLS or SEC
- Determine root cause for unstable gene therapies
- 96-well format for high-throughput testing
- Gather comprehensive data and insight into aggregate particles – size, morphology, counts, distribution
- Get insights quickly with rapid analysis time of 1 minute per sample
- Benefit from a wide working range: measure particles from 1 μm to 5 mm with high reproducibility
- Analyze particles without the interference of buffer or matrix for higher sensitivity
- 21 CFR Part 11 software available
A Better Way to Tackle Lentiviral Stability
With Aura GT, you can quickly identify the most promising lentivirus candidates for late-stage discovery with just 5 μL of material.
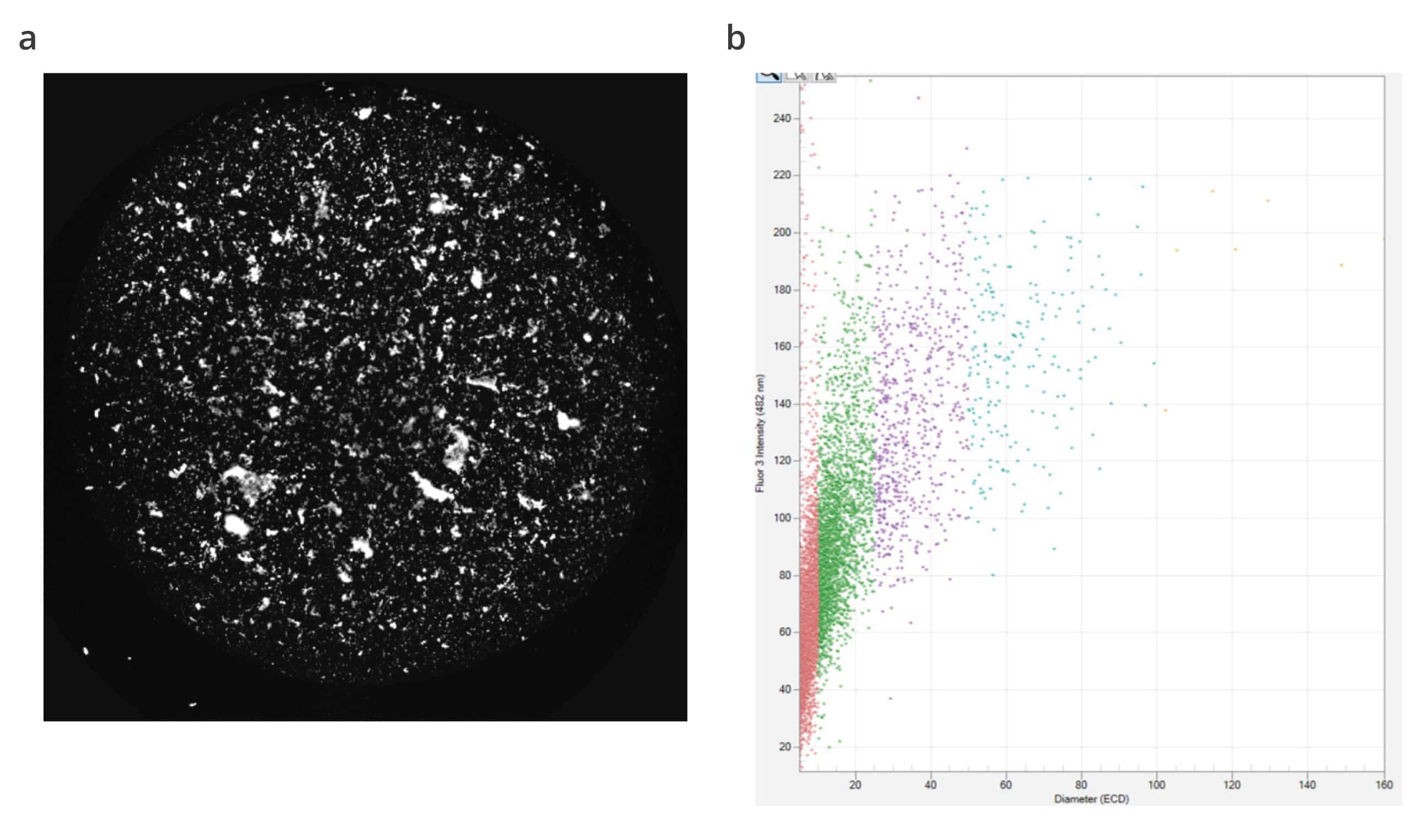
Aura GT employs Backgrounded Membrane Imaging (BMI), a modern form of membrane microscopy that yields quick and accurate results, allowing you to quantify visible and subvisible particles in under 1 minute per sample.
Now you get a comprehensive picture—from images and counts to size distributions and identities. Whether you’re using LVV as starting material for cell and gene therapies or directly as APIs, you can be assured of lentivirus stability throughout the development pipeline.
Get to the Root of Your Lentiviral Vector Aggregation and Instability
Using commercially available dyes like SYBR Gold, Aura GT makes it easy to learn more about the nature of these large lentiviral aggregates.
Fluorescence Membrane Microscopy (FMM) builds upon Aura’s BMI technology to identify biological and chemical makeup. Particle Vue software takes it a step further, delivering characterization and quantification capabilities that enable scientists to get to the root cause of lentiviral aggregation and instability issues.
Featured products
Frequently Asked Questions
The concentration of lentiviral particles refers to the number of viral particles present in a given volume of lentivirus preparation. It is typically expressed as viral genome copies per milliliter (vg/mL) or transducing units per milliliter (TU/mL). Accurate determination of lentiviral particle concentration is essential for dosing and optimizing transduction efficiency in gene transfer experiments and therapeutic applications.
Lentiviral particles typically range in size from approximately 80 to 120 nanometers (nm) in diameter. However, the exact size of lentiviral particles can vary depending on factors such as the specific viral strain, production method, and purification process. Characterizing the size distribution of lentiviral particles is important for understanding their physical properties and optimizing their use in gene transfer experiments and therapeutic applications. Similar to other biotherapeutics like biologics and proteins, lentiviral vectors are also prone to aggregation and instability developing particle sizes ≥1 µm (i.e., subvisible particles).
The titer range for lentivirus refers to the concentration of infectious viral particles present in a lentiviral vector preparation. Lentiviral titer is typically expressed as transducing units per milliliter (TU/mL) or viral genome copies per milliliter (vg/mL). The titer range for lentivirus can vary depending on factors such as the viral vector design, production method, and purification process. A typical titer range for lentivirus preparations used in research or gene therapy applications may range from 10^6 to 10^9 TU/mL or vg/mL.
A good titer value refers to the concentration of a particular substance in a solution, often used in the context of antibody production or viral quantification. For antibody production, a high titer value indicates a high concentration of specific antibodies, which is desirable for their efficacy in various applications such as diagnostics or therapeutics. In viral quantification, a good titer value suggests a high concentration of infectious viral particles, crucial for studying viral behavior or for vaccine development. The specific threshold for what constitutes a "good" titer value can vary depending on the specific application and the desired outcome, but generally, higher titer values are preferred as they indicate higher concentrations of the desired substance.