Step Up Your USP 788 Compliance Game
It's not enough to simply meet the bare minimum requirements for drug development. USP 788 sets a dangerously low bar, but close analysis of particulate contamination is the only way to guarantee safety and efficacy—not just for patients but for manufacturers too.
USP 788 suggests using Light Obscuration (LO) techniques as well as a microscopic particle count test for particulate analysis (USP 788 Method 2). But relying on LO alone isn't enough to get a complete picture when it comes to high viscosity formulations or extrinsic particle contamination.
This calls for a solution that can deliver a precise and accurate technique for characterizing subvisible particles as well as contaminants.
Enter Aura’s industry-leading Backgrounded Membrane Imaging (BMI) and Side Illumination Membrane Imaging (SIMI) technology.
Why Use Aura for Assessing Particulate Contamination?
- Achieve more accurate results with minimal amounts of sample (as little as 5 μL per test) so that multiple measurements can be made and averaged.
- Achieve higher throughput for testing lots of candidates or conditions
- Gather comprehensive data and insight into aggregate particles – size, morphology, counts, distribution
- Get insights quickly with rapid analysis time of 1 minute per sample
- Benefit from a wide working range: measures particles from 1 μm to 5 mm with high reproducibility
- Image particles without the interference of buffer or matrix for higher sensitivity
- Quantitate non-homogeneous particle populations (size, density, morphology)
- Detect changes as a function of stress and solution conditions
- Enjoy a user-friendly software interface
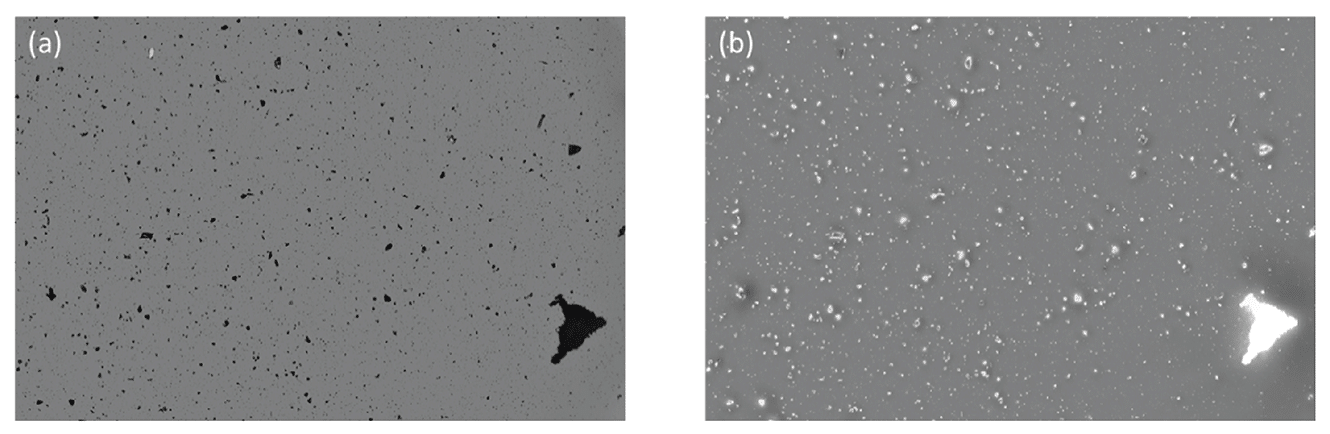
Detect Inorganic Contaminants
Particulate analysis at subvisible and visible levels presents an intricate challenge due to the similarities between biologics, some extrinsics and even other organic contaminants like cells.
Fortunately, solutions such as Aura particle analyzer and Particle Vue software can help make sense of it all.
These powerful tools offer accuracy in sizing and morphological determination of large aggregates.
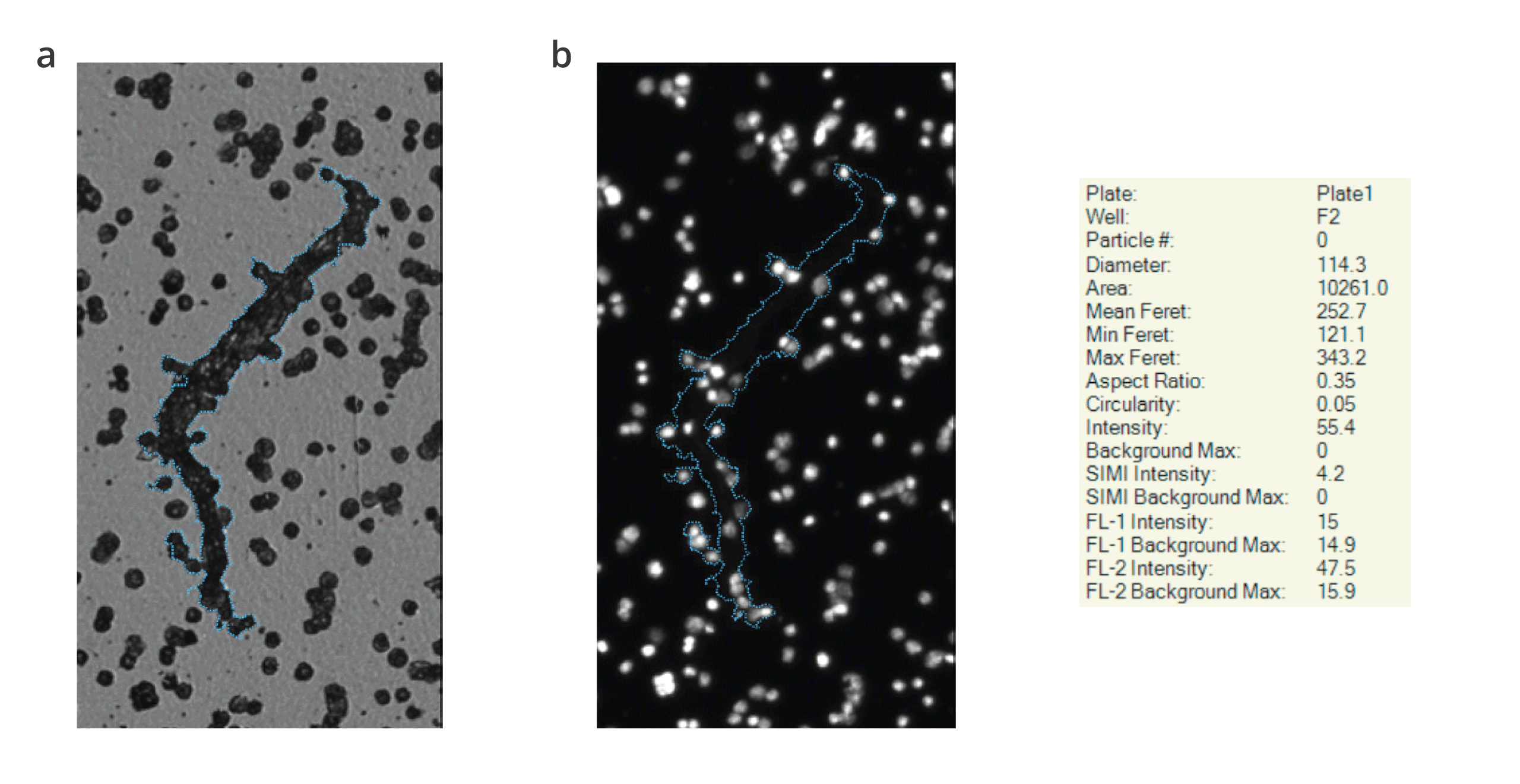
This is thanks to BMI and Fluorescence Membrane Imaging (FMM) technologies, which allow specific particles to be identified so we have a better understanding of the nature and origin of visible aggregates. Comprehensive particulate matter testing is achieved through these advanced testing methods, outperforming light obscuration method and visual inspection, ensuring the highest standards of product purity and safety.
See the Unseen—Experience High-Contrast Particle Imaging Like Never Before
BMI breathes new life into membrane microscopy, replacing the tedious USP 788 subvisible particle lot release method, with modern robotics and automated imaging to generate accurate particle counts and sizing.
Built exclusively on Aura, this innovative technology can perform particle characterization on up to 96 samples in a single run using as little as 25 µL per sample.
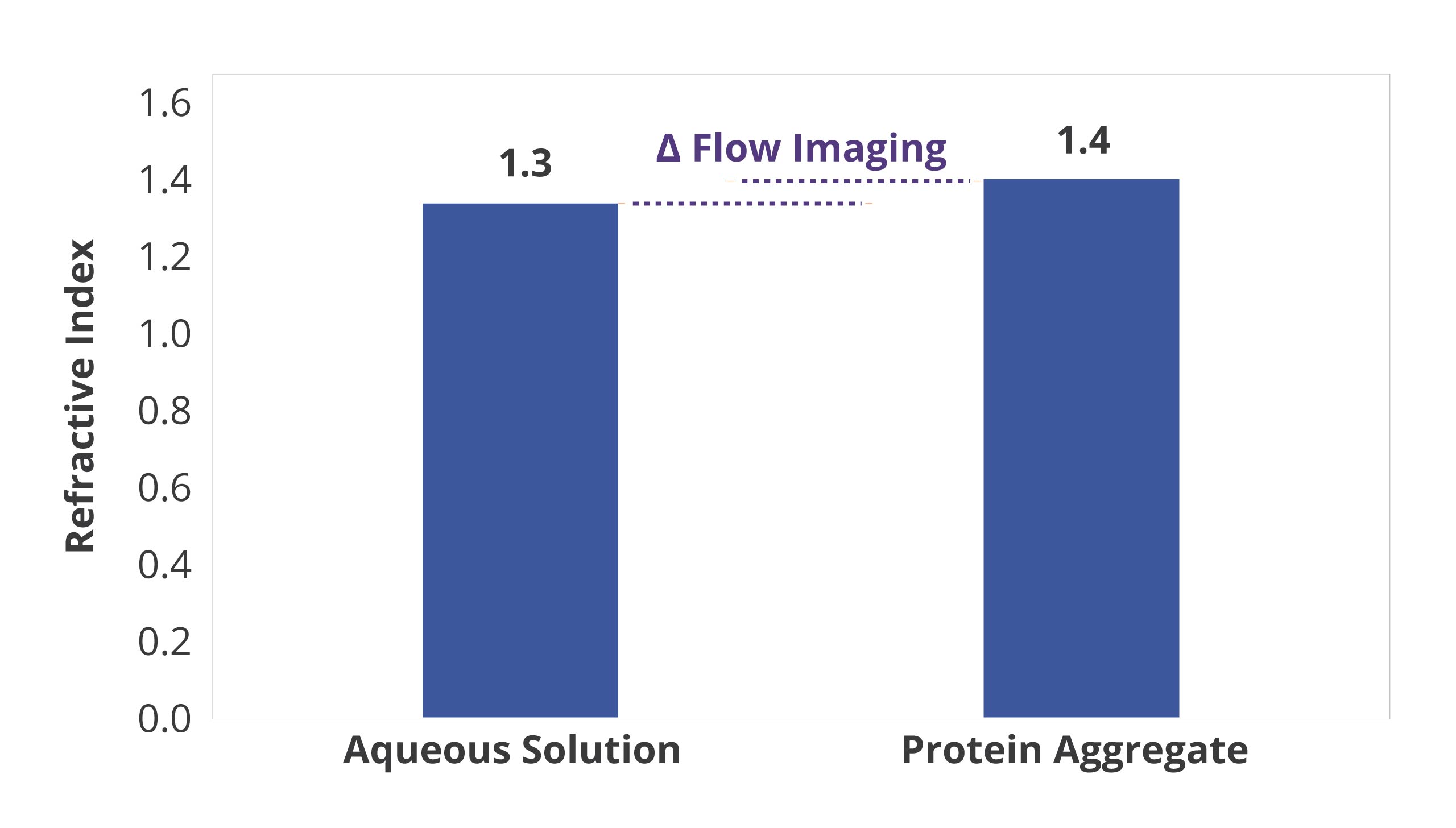
BMI is also insensitive to solution refractive index, enabling reliable measurement of translucent protein aggregates.
The end result: USP788-compliant subvisible particle data, eliminating the need for LO and providing better insight into particulate contamination.
Granular Insights in Every Sample
Quantifying particle counts of historic USP size bins (>10 μm, >25 μm) is integral for accurate analysis, but being able to characterize particles <10 μm as well as determine if particles are intrinsic is just as essential.
With Aura’s SIMI and FMM technology combined with BMI's refractive index capabilities, you can now gain a deeper understanding of samples to identify protein or non-protein contents along with intrinsic or extrinsic elements—something that traditional flow-based particle analyzers could not provide. This allows for greater protection against costly recalls due to unseen particulate contamination.
Frequently Asked Questions
Particulate contaminants in pharmaceutical products can arise from various sources and may include foreign particles such as glass shards, metal fragments, fibers, or microbial contaminants. Common particulate contaminants encountered in pharmaceutical manufacturing include visible particles (>50 micrometers), subvisible particles (1-50 micrometers), and microbial particles (e.g., bacteria, fungi). These contaminants can originate from raw materials, packaging components, manufacturing equipment, or environmental sources. Particulate contamination poses risks to product quality, efficacy, and patient safety, necessitating rigorous quality control measures and analytical testing throughout the manufacturing process to detect and mitigate contamination issues.
Particle count testing in the pharmaceutical industry involves quantifying the number and size distribution of particles present in pharmaceutical formulations or manufacturing processes. This testing is critical for assessing product quality, ensuring compliance with regulatory standards, and identifying potential contamination issues. USP 788 has largely been accepted as the industry standard for particle count testing guidance.Common methods for particle count testing include light obscuration, microscopy particle counting, and dynamic image analysis. These techniques provide quantitative data on the concentration and size distribution of particles, enabling manufacturers to monitor and control particle levels within acceptable limits. The Aura family of instruments can identify, size and count microbial particles easily, accurately and quickly. Particle count testing is particularly important for injectable drug products, where excessive particulate matter can pose risks to patient safety, such as embolism or injection site reactions.
Controlling contamination requires implementing comprehensive quality control measures and adhering to good manufacturing practices (GMP). Key strategies for contamination control include:
- Facility design and maintenance: Designing facilities with controlled environments, proper ventilation, and segregated areas for different manufacturing processes to prevent cross-contamination.
- Personnel training and hygiene: Training on proper gowning procedures, hygiene practices, and aseptic techniques to minimize the risk of microbial contamination.
- Raw material and equipment control: Procedures for the qualification and validation of raw materials, equipment, and packaging components to ensure their suitability and cleanliness.
- Cleaning and sanitation: Establishing robust cleaning and sanitation protocols for equipment, facilities, and production areas to prevent microbial growth and cross-contamination.
- Environmental monitoring: Routine monitoring of air, water, and surfaces for microbial contamination to identify and address potential sources of contamination.
- Quality control testing: Performing analytical testing and inspection of raw materials, intermediates, and finished products to detect and mitigate contamination issues.
- Regulatory compliance: Adhering to regulatory guidelines and standards, such as government regulations, good manufacturing practices (GMP) and pharmacopeial requirements, to ensure product quality and safety.