Stability Matters
We all know the promise that CHO cells hold when it comes to therapeutic antibody development: scalability, high protein titers, and manufacturing potential. Yet, little has been done to characterize the physical stability of secreted antibodies from their inception during CHO cell line development, which leaves you with one option: stabilizing biologics that were not designed with aggregation in mind from the ground up.
For the first time, Aura immunoassays with particle characterization bridge the gap between cell line development and developability to allow pre-screening for antibody stability once mAbs are secreted from CHO cells. Characterization is fast, accurate, and detailed—no more lengthy analysis or prolonged wait times to get the data you need.
Measure Stability, Don’t Just Predict It
Have you been searching for an all-in-one protein aggregation and characterization particle analysis tool that includes advanced formulation characterization? With Aura, now you’ve found it.
Unlike products that simply use protein degradation metrics such as Tm to predict stability, our technology takes it one step further by measuring actual aggregation for stability, providing far more reliable insight into the product quality of your therapeutic antibody. With Aura, now you can truly understand the impact of proper biologics formulation on product stability.
Innovating For Biologics Developers
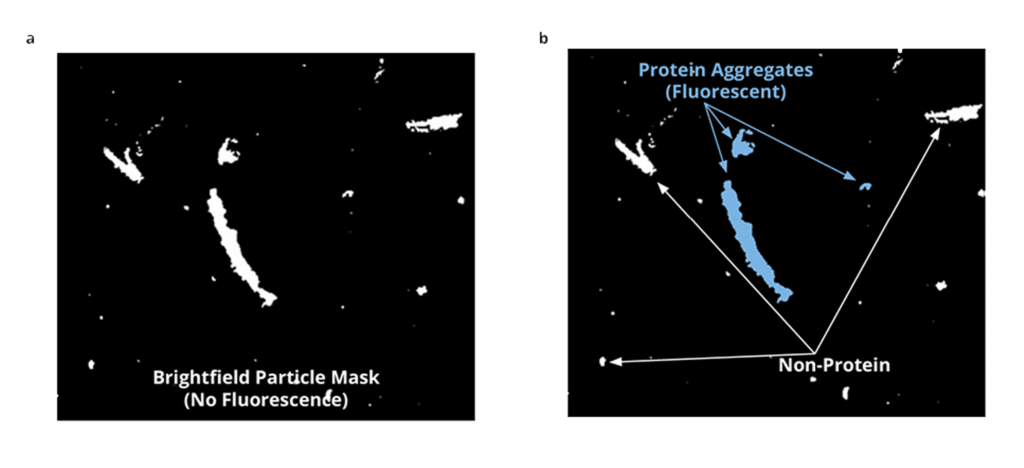
Identifying and quantifying particles is crucial to monitoring stability and promoting long-term efficacy in antibody therapeutic development. But with current analysis techniques, accurate particle identification is impossible. And if you’re using light obscuration (LO), you’re only getting part of the picture and likely missing subvisible particle count all together.
Aura instruments use Fluorescence Membrane Microscopy (FMM) a novel particle identification method that builds on Backgrounded Membrane Imaging (BMI) to identify, categorize, and scrutinize common particles in a sample by using extrinsic fluorescent dyes such as Thioflavin (ThT).
Now you can obtain protein/non-protein ID for a whole 96-well plate assay down to a single individual particle in less than 90 minutes.