Polysorbate 80: Exploring its Impact on IgG Stability and the Importance of Grade and Quality
October 2, 2023
As drug safety regulations intensify, manufacturers must ensure their formulations such as polysorbate 80, are free from visible particles and contain a limited number of subvisible particles larger than 10 µm (as per FDA requirements). These particulates aren’t just due to process instability but also the degradation of formulation excipients.1,2
Mechanical stress during manufacturing can cause protein aggregation, negatively impacting product quality and safety. Aggregation is often the result of weak interactions between exposed hydrophobic patches of partially or fully unfolded proteins. A variety of conditions such as pH shifts, elevated temperatures, and additional mechanical stress can induce protein unfolding, thus promoting the formation of protein aggregates. 1
As a result, developing robust formulations that limit protein unfolding and aggregation is crucial for ensuring protein drug product quality.1
Polysorbates: A Key Player in Preventing Protein Aggregation
To prevent aggregation, polysorbates are frequently used. These nonionic surfactants primarily consist of sorbitan polyoxyethylene (POE) fatty acid esters. The protective function of polysorbates in protein formulations is well-studied, with two primary mechanisms identified: (1) polysorbates acting as a chaperone for aggregation-prone hydrophobic sites on protein surfaces, thus supporting the folded structure, and (2) polysorbates competing with proteins at interfaces, thereby reducing interface exposure.1
Polysorbate 80 (POE sorbitan monooleate, PS80) is a popular surfactant in commercially available biopharmaceutical formulations due to its effectiveness in preventing protein adsorption and aggregation.1 However, there’s a catch: the grade and quality of PS80, particularly during processes generating mechanical stress, can significantly influence protein stability, especially IgG. 1
What’s more, long-term storage of polysorbate at low temperatures can lead to free fatty acid particle formation, potentially compromising drug effectiveness. Traditional analysis methods like light obscuration (LO) and flow imaging (FI) struggle to identify degradation sources. Over time, they can become susceptible to enzymatic hydrolysis by host cell proteins (HCPs), resulting in visible and subvisible particle formation that could compromise a drug’s effectiveness. 1
Conducting high-throughput, sensitive, and specific analysis of polysorbate particles has been difficult due to their complex chemistry and low concentrations formulation environments, making it a perennial needle in a haystack problem.2
Backgrounded Membrane Imaging Used to Explore Functionality of Polysorbate 80
The role of PS80 on protein stability is a topic of ongoing debate. In addition, the impact of partially degraded PS on protein aggregation is not yet well understood. 1 This has led researchers to conduct a study of the functionality of PS80. 1
In the study, a mAb (IgG) was subjected to three different mechanical stress conditions in the presence of multicompendial (MC) and Chinese pharmacopeia (ChP) grade PS80. IgG formulations were supplemented with partly hydrolyzed PS80 to investigate the effect of its degradants on protein stability.1
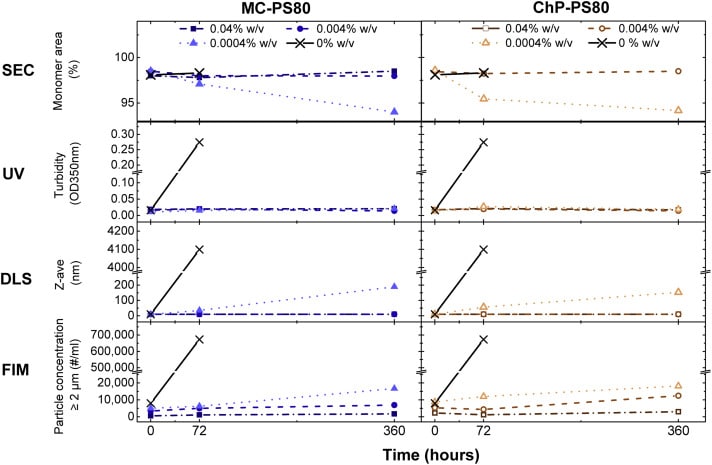
PS80’s functionality was assessed by measuring protein aggregation and particle formation induced during mechanical stress by using several analytical methods. These included: size-exclusion (SEC) chromatography, dynamic light scattering (DLS), flow imaging microscopy (FIM), and backgrounded membrane imaging (BMI). 1
Researchers used Halo Labs’ Aura® to perform BMI for micrometer-sized particle characterization and quantification. Under laminar air flow conditions, 30 μL of the sample was transferred on a polycarbonate 96-well membrane filter plate (Halo Labs) with a 0.4-μm pore size. A vacuum of 350 mbar was applied after filling six wells to allow a flow of liquid through the membrane and entrapment of particles. Subsequently, 90 μL of highly purified water was used to wash each well after sample transfer to remove any soluble material. Each sample was measured in triplicate. 1
The study found no significant differences in PS80 functionality between MC and ChP grades across the stress tests. However, as the degree of PS80 hydrolysis increased, higher counts of subvisible particles were measured after stress. Furthermore, higher levels of PS80 degradants at a constant PS80 concentration may destabilize the IgG. Researchers concluded that MC and ChP grade PS80 were equally protective, but PS80 degradants compromise IgG stability. 1
High-Throughput Polysorbate Particle ID using Aura Assay
Degradation of polysorbate results in three distinct fatty acids: lauric, myristic, and palmitic acid. These acids create particles with unique morphological features that can be pinpointed using Aura’s labeling and imaging capabilities. Through the application of BODIPY™ FL C16, a high-affinity stain, researchers can accurately identify FFAs.
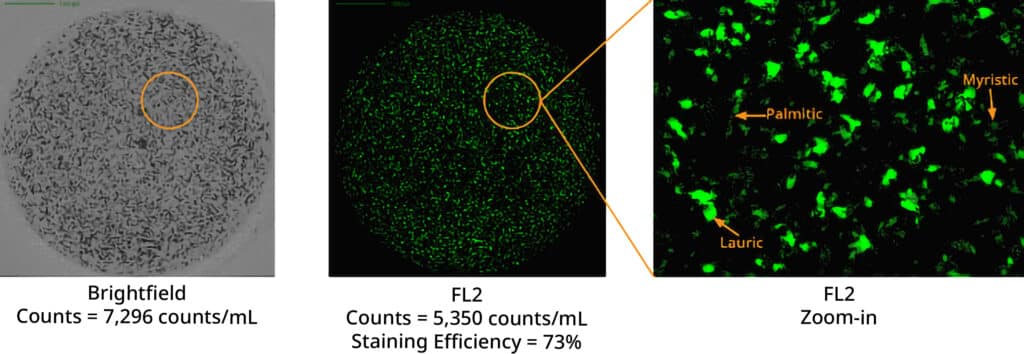
Aura’s polysorbate degradation assay streamlines the process of detecting degraded excipients in protein formulations with elevated concentrations. This method is adaptable, capable of managing a wide range of sample volumes from 5 µL to 10 mL, and can simultaneously analyze up to 96 samples for the existence of free acid particles. This efficiency significantly reduces both time and effort.
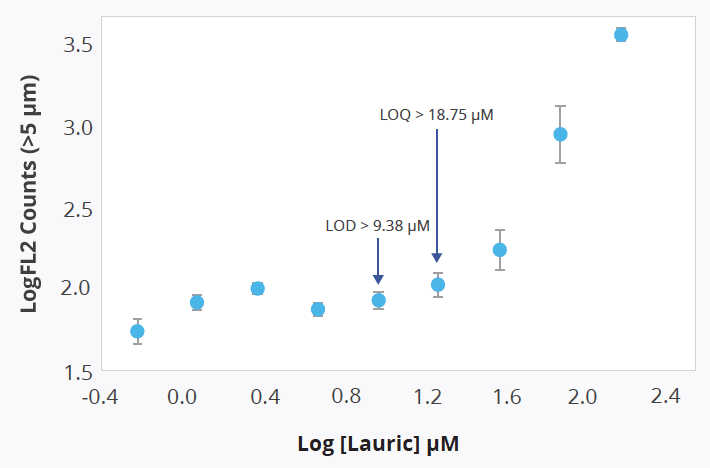
Importantly, the Aura polysorbate assay is sensitive enough to identify free fatty acids (FFAs) at concentrations relative to lauric acid exceeding 9.38 µM (which translates to more than 2183 counts/mL), and can quantify them at levels above 18.75 µM, or over 2976 counts/mL.
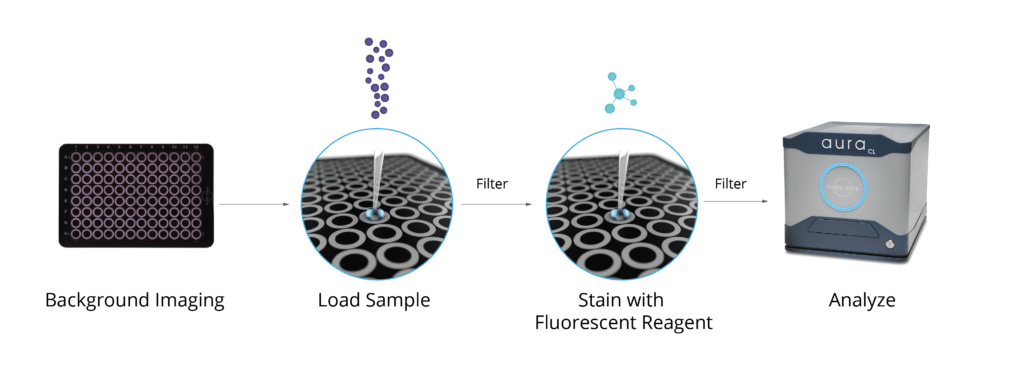
Aura simplifies the detection and distinction of degraded components in protein-based samples at any point in the drug production process. Requiring only 5 µL of sample, this method is both specific and sensitive, capable of analyzing 96 samples in a matter of hours, thereby outpacing alternative techniques.2
In addition, this advanced technology can identify and differentiate crucial degraded particles from polysorbate formulation based on their shape, appearance, and unique labeling with BODIPY FL C16, making it an indispensable tool. 2
Related
- Aura PTx – the instrument for protein and polysorbate characterization
- Check out our poster on protein vs. polysorbate identification and quantitation
- Watch our webinar on how to detect for degraded polysorbate
- Walk through polysorbate detection on Aura with this application note
References
DISCOVER THE AURA FAMILY
Read More ON this topic









