Did you know that subvisible particles (SVPs) are the leading cause of drug product recalls? Despite being tiny, these subvisible particles have a big impact on biologic, cell, and gene therapies – not only limiting a product’s shelf life, but also comprising patient safety. For this reason, injectable drugs face strict requirements to be virtually free of SVPs requiring subvisible particle testing, a method used in pharmaceutical quality control to detect and quantify particles that are not easily visible to the naked eye.
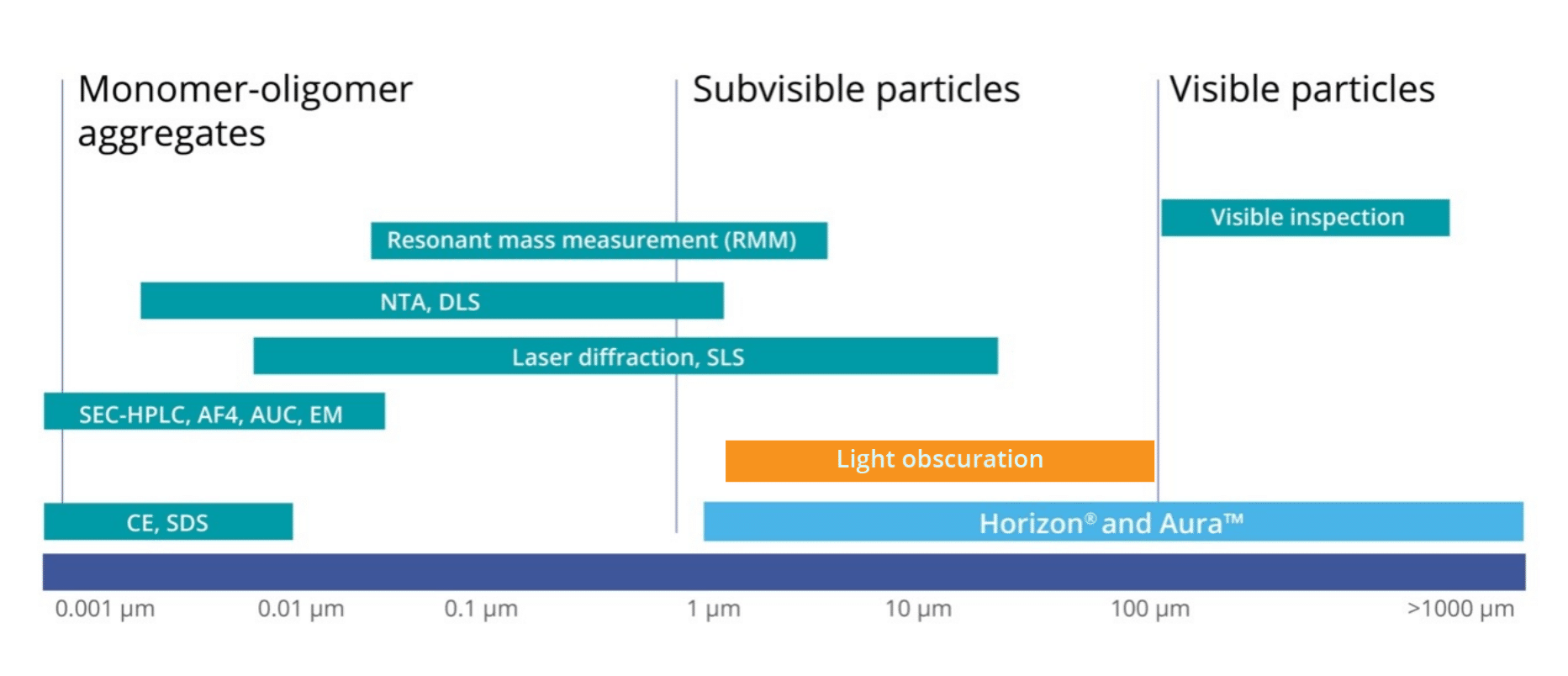
Researchers need a complete understanding and control of physical stability throughout every stage of the manufacturing process. There are a number of factors that contribute to instability and aggregation of biologics including “shear from pumping operations, suboptimal storage temperatures, freeze-thaw cycling, air-water interfaces, and exposure to various surface chemistries.”1 While aggregates can range in both size and solubility, current efforts have focused on insoluble, subvisible particles ranging ~0.1–100 µm, mainly due to the fact that subvisible particles have been shown to “possibly illicit undesired immune responses.”1
Recently, USP 1788 was revised to update and improve USP 788, the primary governing standard for particle analysis of injectables. USP 788 provides an overview of two methods that can be used to determine particulate matter: Light Obscuration (LO) Particle Count Test and Microscopic Particle Count Test. Now, in addition to light obscuration and membrane microscopic methods, USP 1788 provides new guidance on the use of flow microscopy for the determination of particulate matter. USP 1788 revision reinforces the idea that complementary, orthogonal tests must be paired with light obscuration when used alone.
Light Obscuration vs Microscopic Particle Count vs Dynamic Flow Imaging Microscopy
The light obscuration particle counter is an instrument that utilizes a light source and a detector to determine the size and concentration of subvisible particles suspended in a given sample. By passing the sample through a narrow aperture, the instrument measures the degree of light blockage caused by individual particles.
Light obscuration particle count test is currently a common standard technique for the quantification of particles that are ≥ 10 µm and ≥ 25 µm size bins, though it can also measure particles down to 2 µm. However, this method does not come without limitations. It cannot accurately count particles in high viscosity formulations, and also has difficulty assessing particle morphological information.1 Often, particles that are transparent and non-spherical in nature do not effectively obscure light and are, therefore, often undercounted by this method.1
Microscopic particle counting method is viewed as an appropriate technique for the measurement of subvisible particles to ensure safety and control for clinical use. It is a direct counting method that characterizes each sample in its entirety. However, it has been shown to be low-throughput as well as produce result orders of magnitude lower than those reported by light obscuration.1
A third method that is now being increasingly utilized by researchers to quantify and characterize subvisible particles is dynamic flow imaging microscopy. This technique captures dynamic images of particles as they flow through a cell, and then uses those images to calculate the size, shape, count and additional morphological features based on a pixel-by-pixel analysis.1 Yet, this method also has its own set of limitations. The systems used are relatively low-throughput when operated manually, have high sample volume requirements between approximately 200 –700 μL, and consistently have particle carry-over, clogging, or flow cell fouling challenges.
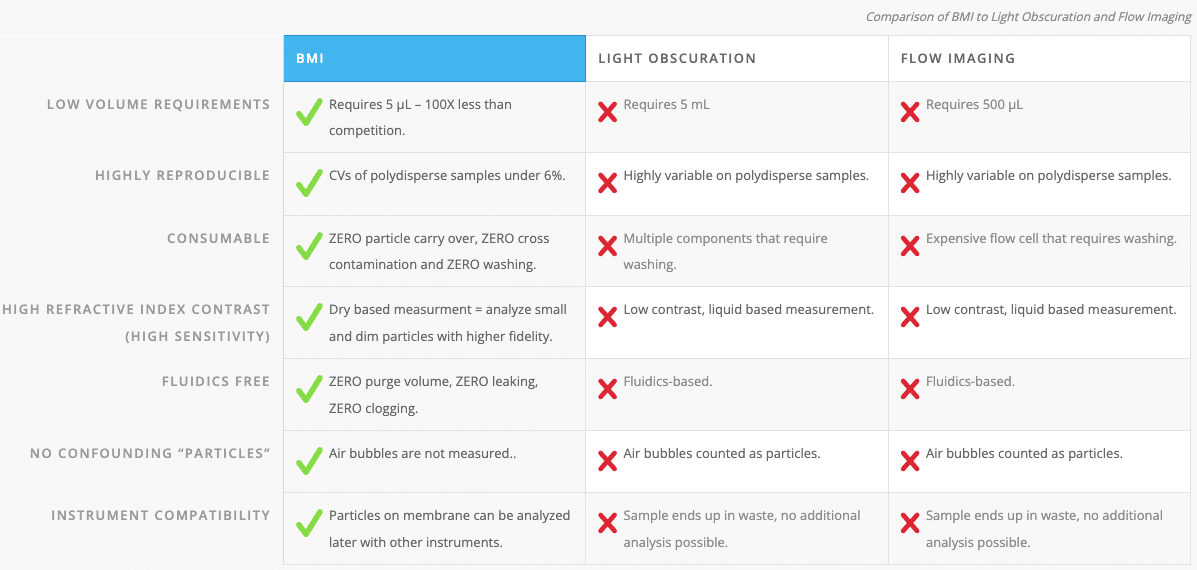
Because of how important it is for researchers to characterize protein aggregates and control their presence in regulatory agencies, it is necessary that they use the most effective tools for monitoring subvisible particle levels to show control and safety.1 Thus, there is a critical need for a dependable high-throughput and low volume technique for subvisible particle testing.
Background Membrane Imaging Provides High Contrast Alternative
Background membrane imaging (BMI) was purposely developed as an alternative technique for the measurement of SVPs. Similar to the microscopic particle count test, subvisible particles from a liquid sample are isolated onto a filter surface for counting analysis by a microscope. The BMI software, Particle Vue, images the baseline prior to particle isolation, and then subtracts that baseline pixel-by-pixel from the isolated particles so that only photographic information from the isolate remains.
This technique has been developed on Aura, the first system that allow particles to be tested both early in development and later during quality control. With Aura, you can determine if a particle is the result of the drug itself, an ingredient in the liquid crashing out of solution, an external contaminant, or even a cell. BMI reinvents membrane imaging with modern robotics, image processing, and novel optics in a 96-well filter plate format that works just like a plate reader.
When developing protein therapeutics, it is critical to have an understanding and control of physical stability during all stages of your manufacturing process. Drug manufacturers can’t rely on minimum standards when they release a product — particles need to be fully characterized every step of the way. And while LO is an effective method, users should not rely on this technique alone. Utilizing BMI with LO will ensure critical therapeutics are safer and developed faster, in order to help patients in need.
Aura systems generate accurate USP 788 compliant subvisible particle data, perfectly complementing light obscuration so drug producers have complete coverage of all particles of interest including subvisible particle testing.2
To have full particle characterization coverage without the LO headaches, switch to Aura today. Learn more about Aura PTx.