Glass delamination has been a persistent challenge in the pharmaceutical industry, posing risks to drug stability and patient safety. The phenomenon occurs when shiny, thin glass layers (lamellae) detach from the interior surface of a glass container and float in the contact liquid.1 These subvisible glass particles (SVGP) can cause serious health risks, including infusion-related complications. Given that delamination often goes undetected until later stages of storage, pharmaceutical companies face potential recalls, supply chain disruptions, and financial losses. The key to addressing this issue is early detection—before visible contamination occurs.1
The Challenge of Detecting Glass Delamination
The instability of borosilicate glass vials—especially in areas with high alkali content, such as the vial’s heel—is the primary cause of glass delamination. During the glass conversion process, high-temperature treatments create chemically uneven surfaces, making some vials far more prone to delamination than others. While the number of recalls peaked in 2011, the issue persists, with up to two product recalls still reported each year.1
Because delamination occurs gradually, waiting for visible glass flakes to appear is not a reliable strategy. To prevent contamination and ensure product integrity, researchers need fast, sensitive, and high-throughput techniques capable of detecting early signs of glass instability before it becomes a dangerous issue.
BMI: The Most Effective Method for Early Detection
Various methods exist for detecting subvisible particles, yet not all identify transparent glass particles like SVGP well. The three primary approaches include:1
- Light Obscuration (LO), as described in USP <788> and Ph. Eur. 2.9.19, measures particles that are at least 10 μm or 25 μm in size and can detect some as small as 2 μm. It works by shining a light through a sample and measuring how much of that light is blocked by particles—the more light a particle blocks, the bigger it is. While commonly used, LO has limitations: it requires a large sample volume, works slowly, and struggles with viscous formulations. It also isn’t effective at detecting transparent particles like SVGP since they don’t block enough light to register.1
- Flow Imaging Microscopy (FIM), on the other hand, captures images of particles as they flow through a cell to calculate their size, shape, and count at the pixel level. Despite providing high-quality images, it’s low-throughput and needs a relatively large sample (at least 200 μL) like LO. Both LO and FIM have difficulty detecting transparent particles like SVGP, making them less reliable for that purpose.1
- Background Membrane Imaging (BMI) on Aura® offers a more powerful solution. This method isolates subvisible particles onto a filter for microscopic analysis, offering high throughput with small sample volumes. More importantly, BMI is better suited for detecting transparent particles, such as SVGP, because the refractive index of these particles are much higher in air than in aqueous buffer. Therefore, BMI can effectively detect transparent SVGP.1 This makes BMI the best option for detecting transparent SVGP.1
Study Compares BMI to Other Detection Methods
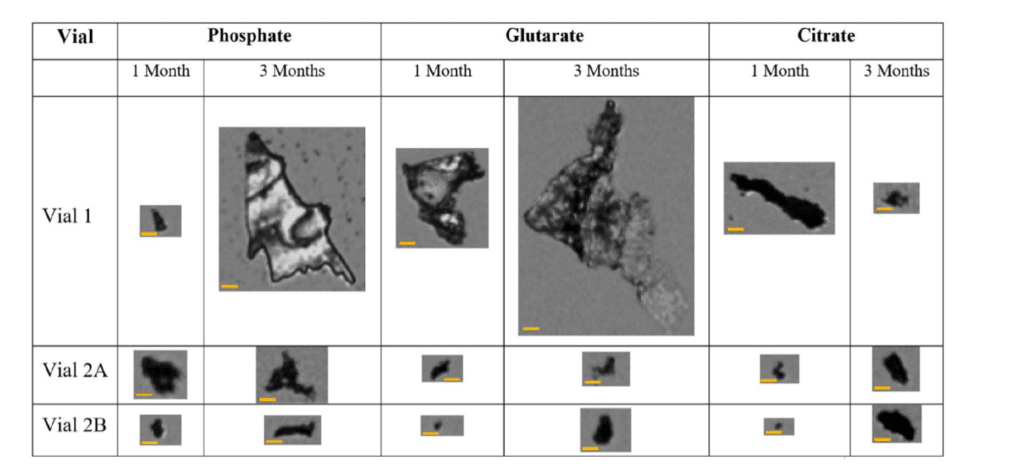
In a recent study, researchers utilized three buffer solutions and examined three glass vial types from two vendors (Vial 1, Vial 2A, and Vial 2B) to evaluate their resistance to glass delamination.1 The study focused on the early stages of delamination, using phosphate (pH 7), citrate (pH 8), and glutarate (pH 8) buffer solutions over one month. A combination of detection techniques was applied to assess the susceptibility of the glass vials to delamination.1
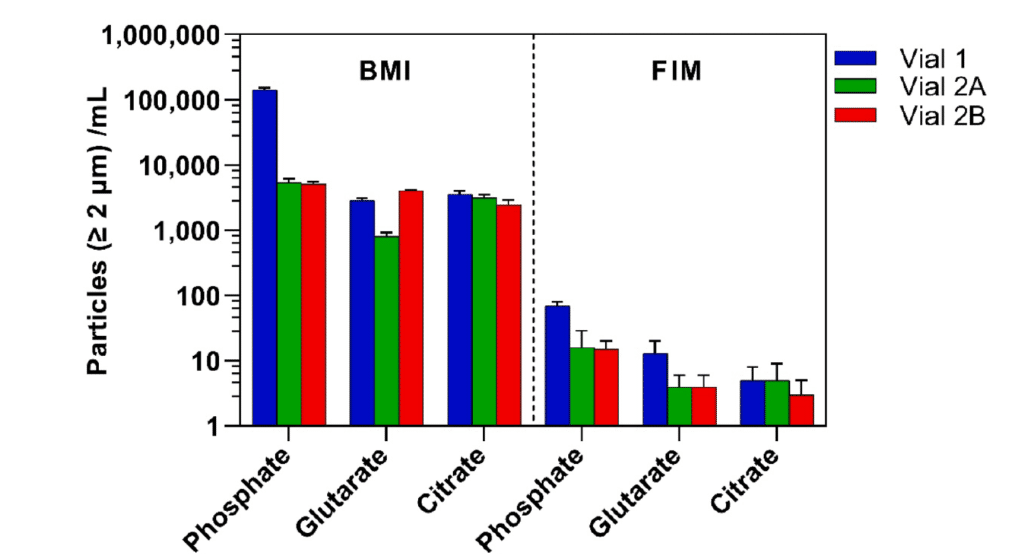
To improve the detection of SVGP, the team leveraged the high refractive index of glass in air rather than in aqueous solutions. BMI was used as a high-throughput and sensitive method to identify SVGP and was directly compared to FIM. Findings revealed that BMI provided more consistent results with other established delamination detection methods. Notably, Vial 1 exhibited a 5–28-fold increase in SVGP compared to Vial 2A and Vial 2B after one month, depending on the buffer type.1
Conclusion
The results of this study highlight the superior sensitivity and efficiency of BMI in detecting SVGP, making it the best method for early glass delamination detection. By analyzing glass vials stored at 60°C for one month, BMI provided stronger consistency with other delamination detection techniques than FIM.1
Key takeaways:1
- BMI detects SVGP earlier than LO or FIM
- Requires smaller sample volumes and offers high throughput
- Provides clearer, more reliable data on vial integrity
By identifying delamination risks before visible contamination occurs, BMI helps pharmaceutical manufacturers make informed decisions when selecting vials that minimize interactions with liquid drug formulations. This proactive approach not only protects drug stability and patient safety but also reduces the risk of costly recalls and supply chain disruptions.1
Related
References
1. Sharifi, F., Maglalang, E., Lee, A., He, J., & Moshashaee, S. (2025). A toolbox for early detection of glass delamination in vials. International Journal of Pharmaceutics, 642, 125236. https://doi.org/10.1016/j.ijpharm.2025.125236
DISCOVER THE AURA FAMILY
Read More ON this topic









