Can a Microplate-Based Method Accurately Screen Formulations for Agitation-Induced Aggregation?
December 10, 2024
The development of biotherapeutics presents unique formulation challenges due to their sensitive nature. These challenges are compounded by the limited availability of material in early preclinical stages, necessitating the miniaturization of formulation studies. This approach not only saves precious materials but also allows access to stability data sooner, shortening development times and enhancing the understanding of factors that affect product stability.1
One critical issue in biotherapeutic stability is protein aggregation, which can impact drug efficacy and patient safety. Triggered by thermal, oxidative, and interfacial stresses throughout a drug’s lifecycle, aggregation is particularly problematic at interfaces where proteins tend to accumulate and form films. Mechanical disturbances, such as those experienced during shipping, can disrupt these films, releasing aggregates that may affect product integrity.1
Orbital shaking tests, which often use large volumes of the drug’s primary containers, are commonly employed to assess biotherapeutics’ vulnerability to these stresses. However, these tests require a substantial amount of material, making them impractical for early formulation testing. Microplates offer an efficient alternative, needing much lower sample volumes, and are compatible with high-throughput devices such as plate readers or liquid handlers. Nevertheless, the efficacy and comparability of microplate-based screening to traditional methods remain uncertain due to existing research gaps and inconsistencies in results from long-term storage studies. 1
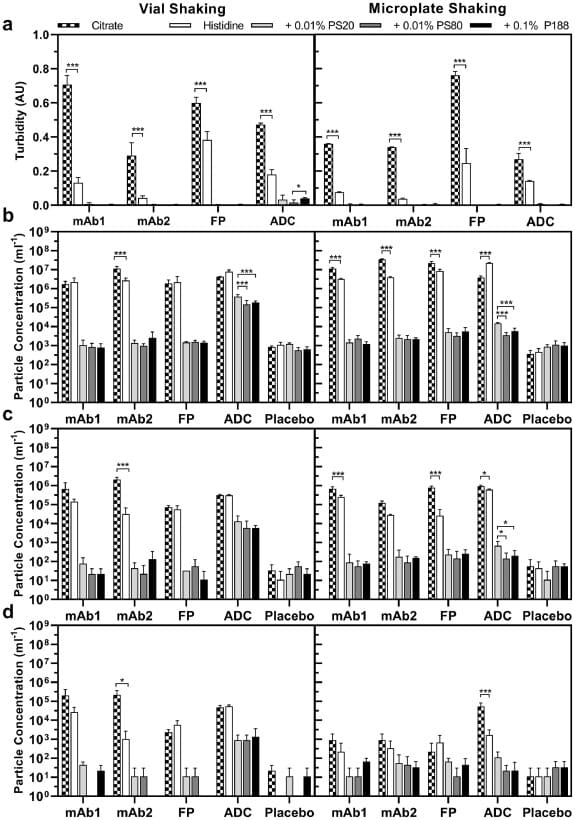
In a recent study published in the Journal of Pharmaceutical Sciences, a team of researchers sought to establish key parameters for a microplate-based method to screen formulations for agitation-induced aggregation. 1
Evaluating the Impact of Orbital Shakers and Microplates
Researchers evaluated various orbital shakers, microplates, seals, and method parameters using an IgG1 monoclonal antibody formulation (mAb1-His) and size exclusion chromatography (SEC). 1
The evaluation criteria focused on measurable monomer loss of mAb1-His by SEC, a homogenous stress distribution over a whole 96-well plate, and seal integrity.1 Key parameters such as shaking frequency, concentration, time, fill volume, well size, shaking orbit, and plate material were studied.1
An optimized microplate shaking method was then applied to discriminate the stabilizing effects of different formulations of two monoclonal antibodies (mAbs), an FP, and an ADC. The results were compared with those obtained from vial shaking studies conducted according to the method described by Eppler et al., using SEC and turbidity. 1
In addition, the study utilized Halo Labs’ Backgrounded Membrane Imaging (BMI) to count subvisible particles above 2 μm. This method ensured that only particles originating from the sample were counted and sized during image processing. 1
BMI Provides High-Throughput, Low-Volume Analysis
Backgrounded Membrane Imaging (BMI) introduces a revolutionary approach to particle characterization, utilizing microscopic imaging on 96-well filter plates for efficient, high-throughput, and low-volume analysis. This technique marks a substantial improvement over traditional methods of particle characterization.
By leveraging advanced image processing techniques, BMI analyzes images to gather detailed particle information. Its working principle is based on acquiring images of custom-designed polycarbonate membrane filter plates before and after sample application. This creates a background image and an actual measurement image, which are aligned and subtracted from each other by image analysis software. The result is an isolated image of the actual particles, ready for analysis.
Conclusion
This study introduced key insights into a microplate-based approach for evaluating biotherapeutic formulations through agitation-induced aggregation. The team concluded that the “good agreement of the presented microplate shaking method to results from vial shaking justifies its use for formulation screening in early development phases.”1 In addition, by employing BMI, the team ensured the exclusion of extraneous particles during image processing, guaranteeing that only those originating from the sample itself were included in the analysis.
Related
- Join our virtual course and gain the skills to load samples, image, count, and size subvisible particles using Backgrounded Membrane Imaging
- See how BMI provides valuable insights into particle analysis
References
1. Florian Johann , Steffen Woll , Matthias Winzer , Jared Snell , ¨ Bernhard Valldorf , Henning Gieseler , Miniaturized Forced Degradation of Therapeutic Proteins and ADCs by Agitation-Induced Aggregation using Orbital Shaking of Microplates, Journal of Pharmaceutical Sciences (2021), doi: https://doi.org/10.1016/j.xphs.2021.09.027
DISCOVER THE AURA FAMILY
Read More ON this topic









