The Invisible Threat: Unraveling the Role of Particle Size Analyzers in Drug Safety
October 10, 2023
Therapeutic protein products are an important weapon in the fight against disease. However, their effectiveness can be compromised if proteins become blocked or inactive due to antibody responses from the body’s immune system. 1
Protein aggregation has been linked directly to these adverse reactions, making it an important risk factor to consider when assessing product quality. 1
That’s where particle size analyzers come in. These instruments are key tools in drug development and manufacturing as they measure the size and distribution of particles in a solution. 1
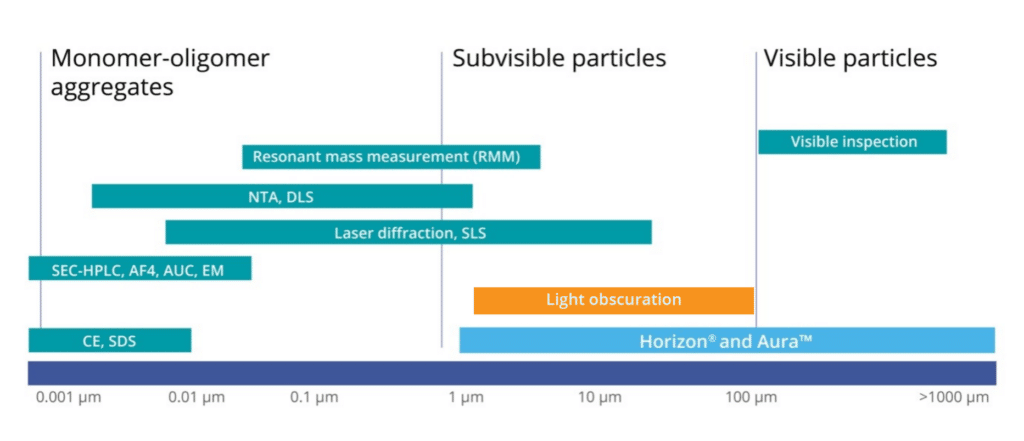
Particles primarily fall into three categories:
- Stable particles in solution (nm sized)
- Subvisible particles (1–100 um sized)
- Visible particles (>100 um)
Among these, subvisible particles (SVP) are the most crucial to detect due to the risk they pose to patient safety. They also act as early warning signs of potential product stability issues, impacting the efficacy of drugs and enhancing immunogenicity. The efficacy of therapeutic proteins can be undermined by an immune response, causing antibody-mediated neutralization of the protein’s activity or changes in bioavailability. Any factor that compromises the efficacy of these crucial treatments can lead to patient suffering and even death. Therefore, it’s critical to prioritize the development and application of particle size analyzers.1
Particle analysis is therefore instrumental in helping pharmaceutical companies adhere to regulatory standards including USP 788, which defines acceptable levels of particulate matter in injections.2 Considering the severe effects these particles can have, the FDA has mandated that injectable drug products need to be virtually free of them.
But how do you determine which particle analyzer works best for your application?
Overview of Particle Size Analyzers
Many techniques exist for measuring particles, each tailored to specific particle types and sizes:
Dynamic Light Scattering (DLS) Particle Size Analyzers
These tools (e.g., Malvern Zetasizer and Horiba nanoPartica) are used to measure particles in the range of ~0.3 nm to ~10 μm. They work based on the principle of Brownian motion, where lighter particles move faster and this speed is directly proportional to particle size. The methodology involves using a laser to illuminate the particles and then analyzing the scattered light and fluctuations in intensity to determine the intensity-weighted size distribution within a sample. However, DLS may not always be the best method for characterization. 3 One of the limitations of DLS particle size analyzers is that they might not detect aggregates in the subvisible range and can be difficult to interpret when samples are polydisperse or if larger aggregates are present. Often the solution to improve data quality is to filter samples before running—which only removes the larger particles so they escape analysis entirely. 4,5
Laser Diffraction Particle Size Analyzers
These instruments (e.g., Malvern Mastersizer 3000 and Horiba Partica LA-960V2) leverage the diffraction patterns produced by a laser beam when it passes through an object ranging in size from nanometers to millimeters. This technique quickly gauges a particle’s geometric dimensions, ranging approximately from 10 nm to 2,000 μm, and it functions independently of the volumetric flow rate, which measures the number of passing particles. However, there are limitations to this method. It requires dilution, which might alter the aggregate size, and it also necessitates a relatively large sample quantity. 4,6
Size and Exclusion Chromatography (SEC) Particle Size Analyzers
This technique is used to evaluate the size distribution of molecules in a sample, typically ranging from 4 to 12 µm. SEC particle size analyzers (e.g., Agilent 1260 Infinity II Bio-SEC Multidetector System) separate molecules based on their size using a column packed with tiny porous beads. However, SEC may fail to detect aggregates that fall in the subvisible range. Other drawbacks include potential matrix interactions, dilution of formulation, and a limited dynamic range for sizes. 4,7
Light Obscuration (LO) Particle Size Analyzers
This standard method can quantify particles ≥ 10 µm and ≥ 25 µm, as well as measure down to 2 µm. Complying with USP <1058> and 21 CFR Part 11 requirements, light obscuration particle analyzers (e.g., Beckman Coulter HIAC) pass a sample through a narrow opening in a device, measuring the light obstruction caused by individual particles to establish their size and concentration. Despite its effectiveness, this technique faces challenges with high viscosity formulations and particle morphological data, resulting in an undercounting of transparent, non-spherical particles. In addition, it requires a relatively large sample volume.4,8
Flow Imaging Particle Size Analyzers
Commonly employed by Yokogawa FlowCam and Bio-Techne (formerly ProteinSimple) MFI, flow imaging microscopy captures particle images in a flow cell to calculate size, shape, count, and other attributes. These standard instruments typically cover a size range from about 1 to 400 µm. However, flow imaging does have drawbacks including possible clogging requiring low viscosity samples, the need for pre-and post-experiment cleaning, low throughput, and high sample volumes (>1 mL).4,9
Light Microscopy
This traditional technique can directly count and characterize subvisible particles in the size range of 0.8–150 µm, as described in both USP 788 and Ph.Eur.2.9.19. Still, manual light microscopy is low-throughput and produces less accurate results than the light obscuration technique.4,10
It’s evident that these legacy particle size analysis methods fail to provide adequate protection, leaving invisible particles unchecked and patient safety in danger.
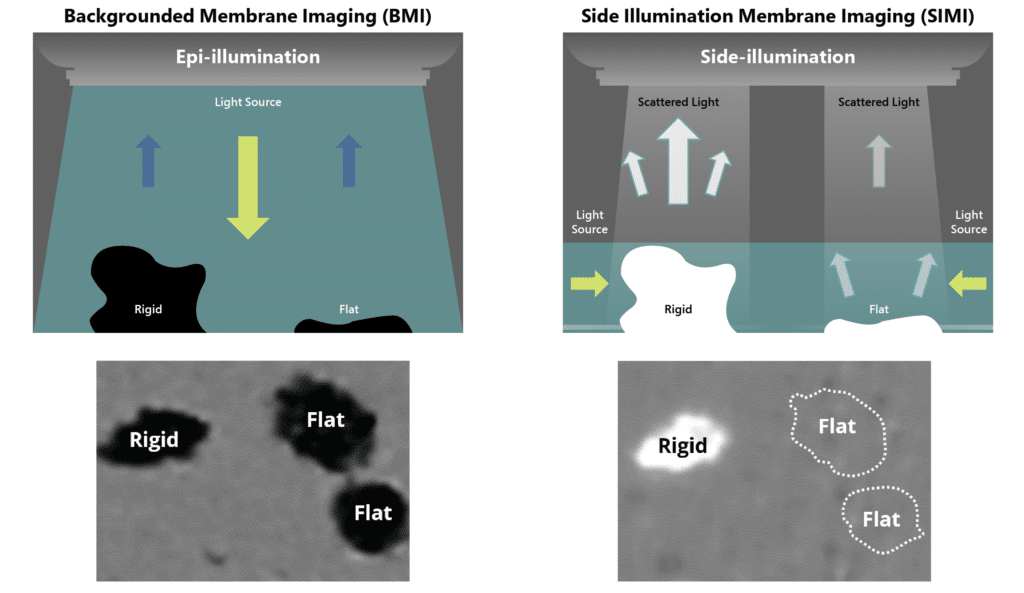
Rethinking Particle Analysis with Backgrounded Membrane Imaging
Aura® particle size analyzers address this critical need to identify and characterize subvisible particles with Backgrounded Membrane Imaging (BMI), a contemporary form of membrane microscopy. Thanks to its high-throughput capabilities, user-friendly interface, and low sample volume requirements, Aura outperforms traditional methods for subvisible particle quantification. It excels in detecting and characterizing particles not measured by conventional techniques such as dynamic light scattering or size exclusion chromatography.
Aura is accompanied by Particle Vue software, an all-in-one software suite that automates particle size analysis and detection in a variety of samples. It provides quick and easy access to particle characterization parameters such as Equivalent Circular Diameter (ECD), aspect ratio, and circularity. This software allows users to view individual particle images, as well as generate interactive scatter plots and histograms to visualize data by multiple characteristics.11
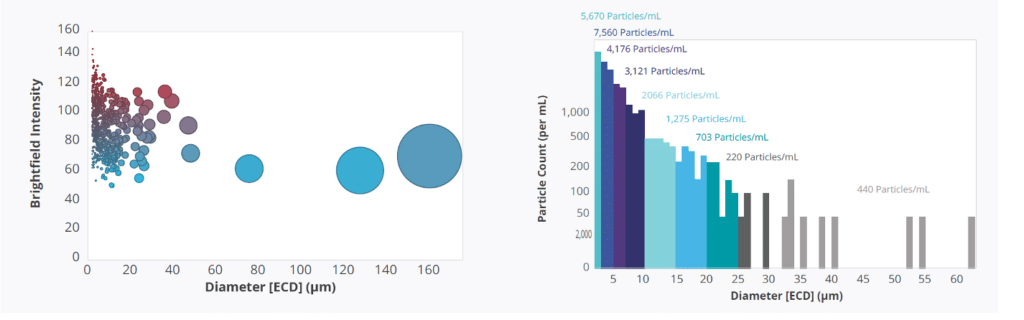
The significance of particle size analyzers in the development of therapeutics cannot be overstated. However, to truly ensure their safety and efficacy before they reach patients, it’s critical to have robust technology capable of thorough particle characterization throughout the development cycle.
With Aura, subvisible particle analysis is possible at the very beginning of your process; a significant leap forward that can facilitate more accurate and clear-decision making. Coupled with Backgrounded Membrane Imaging (BMI) and Side Illumination Membrane Imaging (SIMI), you can be confident that you aren’t missing particles that could prevent costly and life-threatening mishaps.
In addition, it is beneficial to have a range of particle analysis tools to cover the entire spectrum of particle sizes. For instance, having Dynamic Light Scattering (DLS) for nanometer-sized particles, and Aura for micrometer-sized particles, provides comprehensive coverage. This ensures all potential particles, regardless of size, are detected and analyzed, enhancing the quality and safety of the resulting therapeutics.
To learn more about Aura and how our particle size analyzers can help you develop faster, smarter, and safer, visit halolabs.com/aura-particle-analyzers.
Related
- Researching protein and biologics? Aura PTx can be your particle analyzer solution
- Looking at AAVs, LVVs, or exosomes instead? See how Aura GT can provide early particle characterization using ultra-low volumes
- Developing the next generation of cell therapies? Aura CL is just the particle analyzer you need
References
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3928042/
- https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisionGeneralChapter788.pdf
- https://www.sciencedirect.com/science/article/abs/pii/S0065242321000615
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3364383/
- Vargas SK, Eskafi A, Carter E, Ciaccio N. A comparison of background membrane imaging versus flow technologies for subvisible particle analysis of biologics. Int J Pharm. 2020 Mar 30;578:119072. doi: 10.1016/j.ijpharm.2020.119072. Epub 2020 Jan 27. PMID: 32001293.
- https://en.wikipedia.org/wiki/Laser_diffraction_analysis
- https://en.wikipedia.org/wiki/Size-exclusion_chromatography
- https://www.halolabs.com/resource/download-app-note-6-gene-therapy/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3787219/
- https://www.sciencedirect.com/topics/engineering/particle-size-analysis#:~:text=The%20optical%20microscope%20method%20is,%C2%B5m%2C%20and%20down%20to%200.0010
- https://www.halolabs.com/resource/download-app-note-4-sized-right-for-particle-analysis/
DISCOVER THE AURA FAMILY
Read More ON this topic










