Identifying Critical Quality Attributes in Drug Products: Your Key to Ensuring Safe and Effective Therapeutics
August 30, 2023
In drug manufacturing, there is no room for error. Every step and every process must be carefully managed to ensure that a final drug product is both safe and effective. One key concept that plays a vital role is Critical Quality Attributes (CQAs), the foundation of quality control (QC).
In this blog, we’ll take a look at what critical quality attributes are, their pivotal role in drug development, and highlight how our cutting-edge particle analysis technology plays a part in identifying and analyzing these attributes.
Understanding Critical Quality Attributes of Biologics
What are critical quality attributes? A critical quality attribute is a physical, chemical, biological, or microbiological property or key characteristic that needs to be within an appropriate limit, range, or distribution to ensure the desired product quality.1
Critical quality attributes typically fall into three categories:
- Physical Characteristics: these include aspects such as the size and shape of the pharmaceutical product
- Chemical Properties: these encompass elements like the pH level and concentration of the drug
- Biological Attributes: these involve factors such as sterility and bioburden levels in the product
The process of identifying critical quality attributes is an essential step in the drug development process as it ensures that the final drug product is consistent, effective, and safe for patient use.2 Any deviation can influence the efficacy of a drug, potentially leading to harmful effects on the patient.
Moreover, the recognition of critical quality attributes is not just a best practice; it’s a regulatory mandate. Regulatory authorities, including the FDA and the European Medicines Agency (EMA), require the identification of critical quality attributes as part of the drug development process.3 This ensures that drug products meet the necessary quality standards and are safe for consumption.4
The Role of Critical Quality Attributes in Quality by Design (QbD)
Critical quality attributes are at the heart of the Quality by Design (QbD) approach in pharmaceutical development. QbD is a strategy that starts with predefined objectives and emphasizes product and process understanding based on scientific knowledge and quality risk management. In essence, critical quality attributes play a pivotal role in executing a successful QbD approach. They aid in assuring that the end pharmaceutical product is both harmless and effective for patients, satisfying the necessary quality standards stipulated by regulatory bodies.
The Impact of Particle Analysis Technology in Identifying Critical Quality Attributes
In the pharmaceutical industry, technology has emerged as a powerful force in detecting and analyzing critical quality attributes in drug products. One significant critical quality attribute in drug products that has historically posed challenges is the accurate identification of subvisible particles. Measuring roughly 10 µm in size, the presence of these particles can wreak havoc – from shortening a drug product’s shelf life and clogging capillaries to sparking life-threatening immune reactions.
Traditional methods of subvisible particle analysis have struggled to accurately identify these particles, leaving invisible particles unchecked and patient safety in danger. However, thanks to Halo Labs’ Aura® particle analysis, this headache is quickly becoming a thing of the past.
The Aura platform leverages fully automated high-resolution imaging to obtain detailed images of particles within a sample. It allows for the detection and quantification of subvisible particles ranging from 1-100 µm, which are critical quality attributes in various biologics such as vaccines, gene therapies, and monoclonal antibodies.
By utilizing technologies like Backgrounded Membrane Imaging (BMI) and Fluorescence Membrane Microscopy (FMM), Aura can reveal critical subvisible particle data, including count, size, and particle identification. This leads to more precise results and a deeper understanding of the drug product.
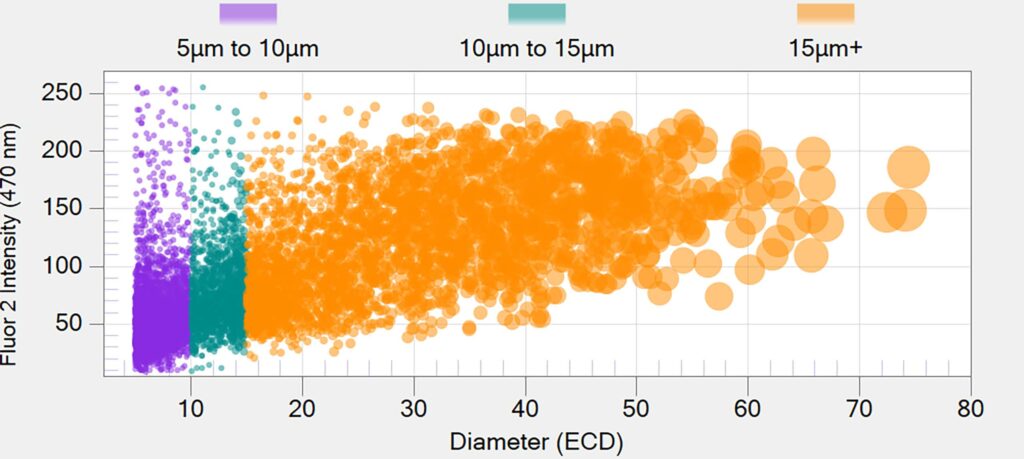
Understanding and monitoring critical quality attributes (CQA) of drug products is not just a beneficial aspect of pharmaceutical manufacturing; it’s a necessity. This key process guarantees that a final drug product surpasses the basic requirement of being effective and ensures the highest standards of safety and quality for patients.
As technology continues to advance, tools like Halo Labs’ game-changing particle analysis technology will play an increasingly vital role in identifying and analyzing these critical attributes.
Related
- Go deeper with critical quality attributes and USP 788
- How does Fluorescence Membrane Microscopy (FMM) work?
- Get the play by play on how to validate Aura for USP 788 compliance
References
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4070262/#CR3
- https://www.linkedin.com/pulse/benefits-identifying-critical-quality-attributes-cqas-mo-heidaran-2e/
- S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry: Q8(R2) Pharmaceutical Development. November 2009.
- https://www.roosterbio.com/blog/critical-quality-attributes-cqas-know-their-importance-limitations-in-product-process-development/
DISCOVER THE AURA FAMILY
Read More ON this topic









