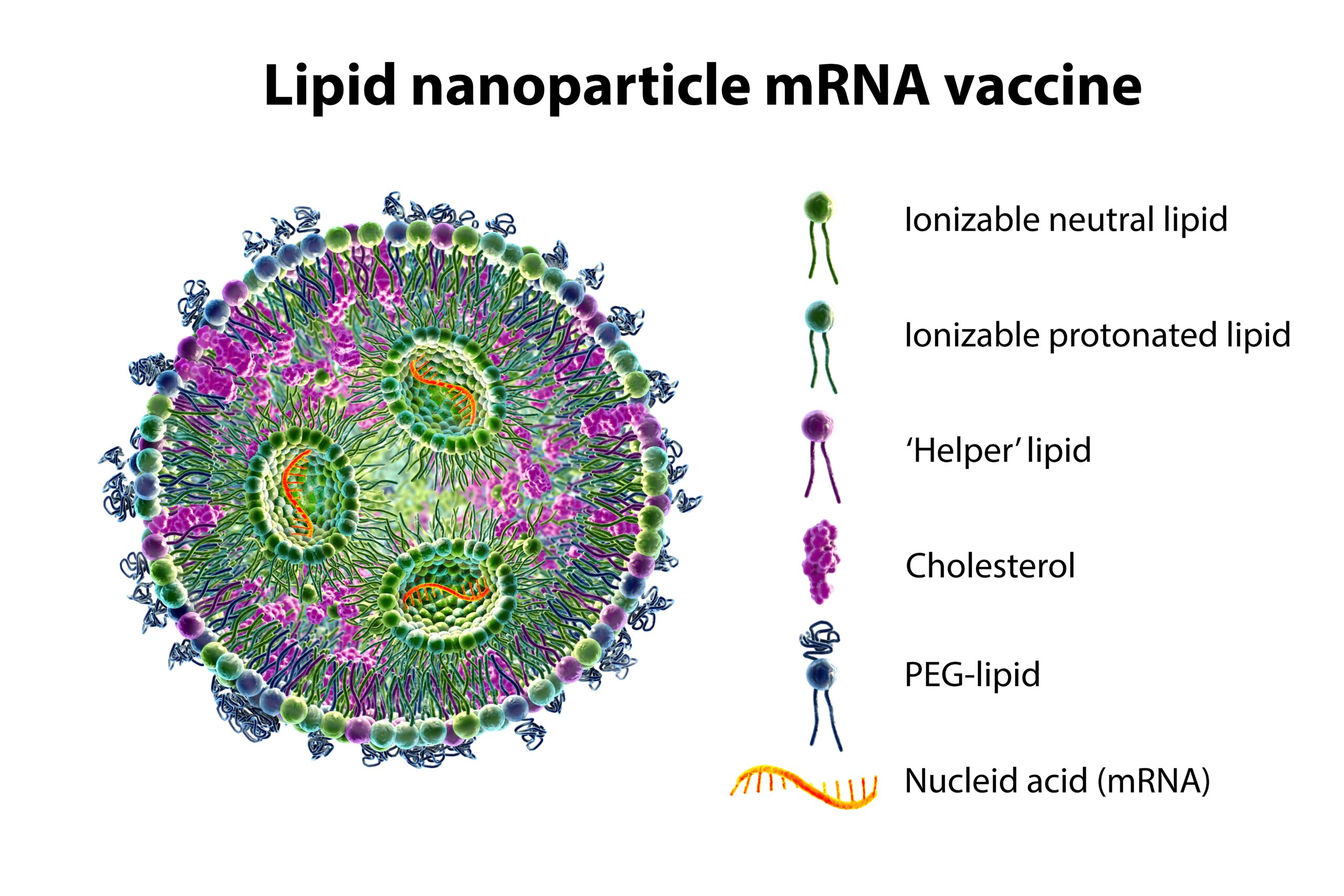
Get the Right Lipid Nanoparticle Formulation Ensuring Effective Drug Delivery
September 13, 2023
In the fight against COVID-19, one technology has quietly transformed the way we combat this deadly virus—lipid nanoparticles (LNPs).
These tiny vesicles serve as an ideal vehicle for genetic material, encapsulating and ferrying it directly into our cells.1,2 They act as a shield for mRNA, protecting it from degradation in the body while ensuring its delivery to the right location in our cells.
In the context of COVID-19 vaccines, mRNA carried by LNPs instructs our cells to produce the spike protein found on the SARS-CoV-2 virus. This process triggers an immune response without causing disease, thus preparing our bodies to fight off the actual virus if encountered.1,3
This innovative use of lipid nanoparticle formulations has become instrumental in the development of mRNA COVID-19 vaccines by Moderna and Pfizer. The reasons for their popularity are clear:
- They encapsulate genetic material within lipid vesicles of sub-micrometer size.
- They can deliver large biological payloads.
- They exhibit low immunogenicity, reducing the risk of adverse immune reactions.
- They are compatible with large-scale manufacturing processes, enabling rapid vaccine production.
However, like all biologics, LNP formulations can be physically unstable, aggregate, and form subvisible particles (SVPs). As a result, proper lipid nanoparticle formulation and evaluation for both physical and chemical stability is required.
Lipid nanoparticle stability refers to the ability of lipid-based nanoparticles to maintain their structural integrity and prevent degradation or lipid nanoparticle aggregation over time. These nanoparticles, composed of lipids such as phospholipids and cholesterol, are widely utilized in various fields including drug delivery, gene therapy, and diagnostics. Achieving lipid nanoparticle stability is crucial to ensure their efficient and safe performance in these applications. The stability of lipid nanoparticles can be influenced by factors such as lipid composition, particle size, surface charge, and environmental conditions. Researchers are constantly investigating novel strategies to enhance stability, including the use of stabilizing agents, optimization of formulation parameters, and surface modification techniques. Understanding and improving lipid nanoparticle stability is imperative for the development of effective and reliable nanomedicines and biomedical technologies.
Redefining LNP Formulation: The Power of Backgrounded Membrane Imaging
USP 787 and USP 788 subvisible particle testing is a key process in assessing lipid nanoparticle formulations. However, Method 1 of USP 788 testing, known as light obscuration (LO), is considered unsuitable for measuring complex solutions such as liposomal and colloidal suspensions. This limitation arises from LO's inability to handle complex matrices effectively. Therefore, USP 788 recommends membrane microscopy (Compendial Method 2) for measuring liposomal formulations. 4
Backgrounded Membrane Imaging (BMI), a contemporary form of membrane microscopy, stands out for its efficacy in characterizing subvisible particles with high throughput and low volume. Aura® uses this method, leveraging BMI's capabilities for comprehensive and efficient particle characterization.
Understanding the Effects of LNP Formulation and Stress on Product Stability
Stress factors, including changes in temperature, pH, and mechanical forces, can significantly impact the stability of biopharmaceutical products,1 which can lead to protein aggregation, compromising the efficacy and safety of a product.5
For example, significant heat stress can break a liposomal formulation apart, increasing lipid nanoparticle solubility and thereby resulting in less insoluble particles captured on the membrane. Conversely, freeze thawing produces more insoluble lipid nanoparticle aggregates, increasing subvisible counts as shown in BMI, exhibiting similar behavior to heat induced aggregation as in most protein formulations.4
In a recent study, we analyzed two lipid nanoparticle samples, each formulated in different buffers. These samples were then subjected to temperature and freeze thaw stresses using Aura GT’s SYBR™ Gold assay to help characterize nucleic acid escape in stressed lipid nanoparticle samples and quantitatively analyze their stability and purity.4
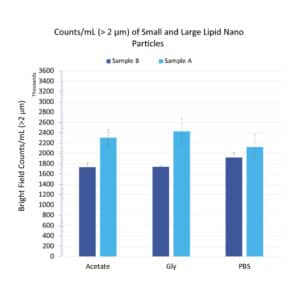
Figure 1. Subvisible particle counts (>2 μm/mL) for Sample A and Sample B treated with varying buffers
Figure 1 shows the >2 μm/mL subvisible particle concentrations for Sample A and Sample B formulated in three separate buffers: acetate, glycine and PBS. All particle measurements showed significant particle concentrations (>1.5E6/mL) across all conditions, with good repeatability (CVs <15%). In addition, both samples exhibited subvisible particle formation that remained consistent and did not vary beyond measurement uncertainty with buffer type. 4
The Link Between Nucleic Acid Release and Coverage of Stressed Samples
It can be challenging to accurately predict the quality of lipid nanoparticle drugs, even when using samples from well-known commercial developers. Every formulation has its own particle profile, and LNP formulations are no different. This is a common theme in the stability of subvisible particles.
Leveraging our SYBR Gold assay via Fluorescence Membrane Microscopy (FMM), Aura GT solves this issue by assessing both the stability and purity of samples in high throughput, low volume testing, enabling accurate LNP characterization at earlier stages, from initial development all the way through to final product release.
In Figure 2, we take a deeper look at subvisible particle images for Sample A before applying stress and after freeze thawing. The freeze thawed membrane images are shown in three imaging modes (2a) BMI, (2c) SYBR Gold fluorescence and (2e) combined BMI and SYBR Gold fluorescence image, whereas the unstressed sample images are in (2b) BMI, (2d) SYBR Gold fluorescence and (2f) combined BMI and SYBR Gold fluorescence images.
Both sets of BMI membrane images show significant subvisible particle formation for unstressed and stressed types. Upon staining both the unstressed and freeze thawed samples with SYBR Gold, that the stressed sample shows significant area portions of strong SYBR Gold fluorescence, indicating insoluble samples that are coated with nucleic acid material, whereas no fluorescence signal is visible in the unstressed sample. 4
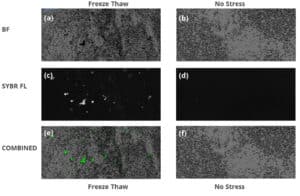
Figure 2: Subvisible particle images of no stress and freeze thawed conditions for Sample A. Blue scale bar is 100 μm long. Freeze thawed condition (a) BMI, (c) SYBR Gold fluorescence, and (e) combined BMI and SYBR Gold images. No stress (b) BMI, (d) SYBR Gold fluorescence, and (f) combined BMI and SYBR Gold fluorescence images.
Importance of Proper Lipid Nanoparticle Formulation
Freeze thaw cycles can significantly increase subvisible particle formation, while heat stress can significantly reduce the subvisible particle concentration across both samples. Compared to protein formulations, this heat stress effect may appear unconventional. However, considering the lipid nanoparticle composition and upon further look at the literature, we are able to understand this phenomenon and conclude that significant heat stress can break a liposomal formulation apart, increasing the LNP solubility and thereby resulting in less insoluble particles captured on the membrane.
On the other hand, freeze thawing produces more insoluble aggregates, thereby increasing subvisible counts as shown in BMI, exhibiting similar behavior to heat induced lipid nanoparticle aggregation as in most protein formulations. A recurring theme in subvisible particle stability is that each formulation exhibits its own particle profile, LNPs are no exception. Another interesting data point is that freeze thawed LNPs showed significant SYBR Gold staining, whereas unstressed LNPs did not, indicating a unique nucleic acid leakage or rupture behavior for this type of stress.4
Conclusion
The use of LNPs as a drug delivery technology is a sophisticated process and requires the best formulation coupled with careful evaluation to ensure their efficacy is not compromised.
Low-volume, subvisible particle measurements must be accurately taken to properly assess LNPs for stability. However, traditional methods may not give you the accuracy, speed, or flexibility you need.
Aura GT solves these challenges, providing a solution to subvisible particle analysis of lipid nanoparticles, from early-stage inception through to product release.
DISCOVER THE AURA FAMILY
Read More ON this topic