Cell culture contamination is the bane of every laboratory’s existence. They can be physical, chemical, or biological, ranging from minor problems to catastrophes.
Solutions to contamination range from observing good laboratory practices, maintaining high standards of cleanliness and care of culture media, effective cleanups, and using technologies like Halo Lab’s Aura+ particle characterization instrument to detect and characterize the contaminants, and potentially the source of contamination and other remedies.
This analysis will review the significance of contamination control, contamination types, identification testing of particulate contamination, and various detection and control methods.
Overview of Cell Culture
Cell culture—the practice of growing and maintaining live cells in an artificial laboratory environment—is one of the most important techniques in cell and molecular biology. Cell cultures enable researchers to discover the biology, chemistry, physiology, and metabolic processes in wild-type, diseased, and modified cells.
Immortal cancer cells have shown researchers how cancer develops (and can be treated or prevented), and stem cells derived from individuals with inherited disorders have become valuable platforms for studying the molecular basis of disease, all in a dish. Cell cultures are also used to screen for new drug compounds for efficacy and toxicity in certain cells and are invaluable for developing regenerative tissues, vaccines, and gene and cellular therapies.
Cell Culture Contamination Control
Depending upon the contaminant in question, the US Food and Drug Administration (FDA) and American Type Cell Collection (ATCC), estimate that up to 30 percent of all cell cultures are contaminated (at least with mycoplasma).
Consequences of cell culture contamination include delays in experiments and cell growth, and financial setbacks from having to purchase new cells, culture media, sera and dishes/vessels, and labor costs from having to re-set up experiments and develop new cultures.
For drug development, hidden contaminants can alter the growth and function of your cells, and their ability to produce biological therapies and vaccines. Such products are often rendered useless and in violation of drug safety regulations and standards.
Total prevention of cell culture contamination may not be possible, but particle and physical contaminants can be held to a minimum, with the right strategies and tools in place to prevent losses of cultures, experiments, and production. This type of contaminant may be considered the biggest concern as it could put patients at risk or even death.
“For biotherapeutic production, USP <788> standards set biopharmaceutical lot release guidelines and the proper limits of subvisible particles of no more than 12 particles per ml at least 10 µm and no more than two particles per ml that are at least 25 µm. (in biotherapeutics, these particles are usually protein or other molecular fragments). The Halo Labs Aura™ system is an invaluable tool in identifying and characterizing subvisible particles behind some cell culture contamination.
Contamination Risk Assessment
Determining the risk of contamination of your cell culture hinges on a thorough evaluation of the properties of your culture. These properties are intrinsic—what sera and media are you using, and what properties cells have after genetic modifications—and extrinsic—what is the possibility of infection with pathogens, other protein particles, and even cross-contamination from other cultured cells (HeLa, for example).
Some cultures present higher risks than others:
Lower risk—using non-human or non-primate, continuous cell lines, or well-characterized human continuous cell lines.
Medium risk—using poorly characterized mammal cells.
High risk—using primary cells from human or primate tissue and/or blood. High risk cells are also those that contain endogenous pathogens.
You also will want to conduct a thorough review of your laboratory procedures to make sure Good Laboratory Practices (GLP) are followed, proper disinfectants are deployed regularly, cell cultures are inspected for possible infection/contamination, and the proper tools are in place that can detect and quantify invisible or subvisible agents.
Types of Contamination
Contamination can arise from many different sources. For most researchers and developers, bacteria and fungi usually come to mind first as common contaminants. Other types can include viruses, chemicals, and macromolecular particles.
Microbial contaminants—these are usually plainly visible, with rapid turbidity and color changes in media. These include bacteria, fungi and yeast. Other intruders, such as mycoplasma, are not as easy to detect, however.
Viruses—these are some of the most difficult-to-detect contaminants. Viruses can come from the patient or host animal, and some biotechnology cell lines contain endogenous retroviruses.
Chemical contamination—a non-living contaminant, including free radicals, metal ions, residues from disinfectants and detergents, or leftover bacterial endotoxins. For biotherapeutics and vaccine developers, these contamination agents can also include protein fragments and other larger molecules that accompany protein-based drug/vaccine manufacturing and are a significant problem for biologics developers.
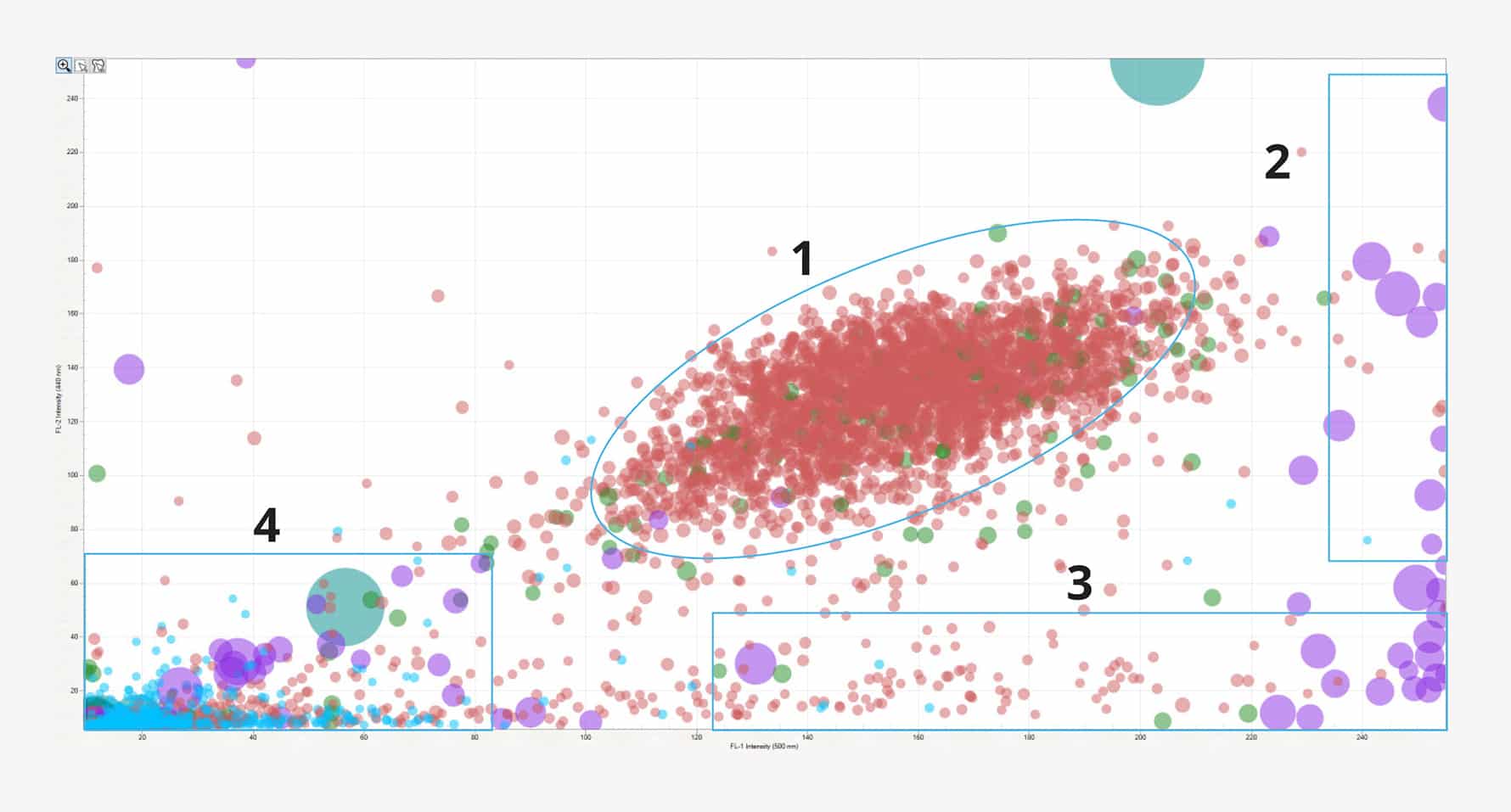
Identify cell doublets and triplets (1), cellular aggregates (2), protein aggregates (3), and plastic contaminants (4) in a cell therapeutic sample.
Contamination Analysis of Trace Chemicals, Metals
Depending on the type of contamination and cell culture being used, contamination analysis can employ several techniques and tools. These tools detect, identify, and characterize contamination and extraneous matter in cell culture. Analytical tools can range from visual inspection, microscopy, spectroscopy, physical/chemical investigation and element analysis. Rapid detection and analysis are key.
While microscopy (optical, confocal laser scanning, electron) can provide information on the shape, size, and surfaces of contaminants, spectroscopy and other microscopic techniques (scanning electron microscopy and energy-dispersive X-ray spectroscopy and determine the chemical composition of a contaminant. Raman spectroscopy, fluorescence microscopy, optical, and ATR-FTIR spectroscopy provide molecular composition of organic materials, while inductively coupled plasma spectroscopy can detect trace metals.
Contamination Analysis—Identification Testing
Determining chemical and inorganic contamination can be difficult because these contaminants are more difficult to detect than biological contaminants. For identification testing, start by looking at any changes in the lab that happened weeks before the problem arose, especially changes in equipment, supplies, solutions, and media. Brainstorm with laboratory personnel and determine the best direction for further evaluation. It’s also important to try and match what could happen, especially given the risks of your cell culture.
Identification can begin by recording any “off” odors or flavors (if applicable), off-color issues, or foreign particles. Rule out (if possible) contaminants from improper storage and handling, or poor-quality raw materials. Then, you can start using tools that can identify a chemical, physical, or biological contaminant.
Particulate Contamination Analysis
Particulate matter, especially subvisible particles (2 to 100 µm), can be common in biopharmaceutical injections and infusions and are strongly linked to immunogenicity, and thus, product impairment or failure.
USP <788> and FDA standards and regulations establish lot release guidelines and limits for these particles. USP <788> describes two methods for particulate analysis—light obscuration and membrane microscopy. Membrane microscopy is recommended over light obscuration when working with complex, viscous and low-volume samples (a large proportion of biopharmaceutical drugs under development).
Once microscopic particles have been identified, you can then move to isolate the particulate phases of production deposits and aggregation and determine the sources of the contaminants. These particles can be protein fragments, but can also be extraneous particulates that appear as black specks, cleaning and maintenance residual chemicals, particles seen after dissolution, metal abrasion or corrosion from pipes, pumps or containers, process equipment particles, glass fragments from delamination or breakage, minerals, or various plastics, rubber, silicone or other polymers.
Contamination Analysis Techniques
Determining the best particle analyzer depends on specific application requirements, such as sample type, size range, and analysis objectives. There isn’t a one-size-fits-all solution. However, popular particle analyzers often utilize laser diffraction, dynamic light scattering (DLS), or microscopy-based techniques. The Aura suite of analyzers from Halo Labs can provide more detailed particle analysis than DLS or microscopy for particles ≥1 µm, offering BMI and FMM to find and characterize more particles, including subvisible particles that otherwise go undetected.
Particle Size Analysis
Nearly all biotherapeutics contain subvisible particles (between 2 and 100 µm in diameter). The most important property of particulate samples is particle size, which can be crucial to the success of your final product. Most particles vary in volume and shape. Understanding the particle size distribution in your product can help you make predictions about its manufacturability, efficacy, quality, bioavailability, and shelf life. Size distribution can have an impact on the porosity and surface area of your therapeutic—the wrong particle sizes can interfere with production, yields, and profits.
Detection Methods
Laser diffraction—also known as static laser light scattering, this method measures size indirectly by detecting intensity distributions of laser light scattered by particles. Shape evaluation is not possible (it assumes all particles are spherical), and low resolution and sensitivity are disadvantages. It is not as effective in polydisperse samples.
Polymerase chain reaction (PCR)—this popular technique can be used to determine the species of biological contaminants, such as mycoplasma, viruses, or bacteria.
Dynamic light scattering (DLS)—in this method, particles move past a camera and are analyzed in real-time. DLS can examine millions of particles in minutes and report a variety of size as well as shape parameters. Currently, DLS is the most commonly used method for biotherapeutic particle size distribution analysis.
Imaging (microscopy, SEM)— here, particles are measured by comparing their size against a grid of lines and counting them. However, millions of particles need to be measured to achieve a statistically valid analysis. Automated analysis of electron micrographs helps ease this barrier. This method is useful for particle sizes within 0.2 to 100 µm.
BMI/FMM—backgrounded membrane imaging, combined with fluorescence membrane microscopy, are the basis of Halo Lab’s Aura particle analysis system. BMI, a high-contrast imaging technique, develops clear pictures of particles in a sample. It only requires 5 µl of sample and can deliver results in a minute. FMM labels particles using specific fluorescent dyes or antibodies, resulting in simple and definitive identification.
Comparing Methods
Microscopy—light, electron, X-ray diffraction—can illustrate the size and shape of contaminants but may not be able to detect subvisible particles. PCR is sensitive, but cannot detect protein fragments or chemical/physical contaminants. Backgrounded membrane imaging (BMI), combined with fluorescence membrane microscopy (FMM), and side illumination membrane imaging (SIMI), the foundation of the Aura particle detection system, can easily, quickly and accurately distinguish particles in cell, gene and protein therapies.
BMI—brings clarity to formulation risks that were previously hard to see. It has roots in membrane microscopy, but with significant advances. A background image of the membrane is first taken before samples are filtered through and particles captured. The same membrane is imaged again, with particles on the surface. Then, subtract the background image so the background texture is eliminated, revealing the particles.
FMM—works with BMI for a level of analysis not seen in other particle analysis systems. Label your targets with specific fluorescent dyes or antibodies, either on the membrane itself or in solution. First image the membranes with BMI to locate particles, then use FMM to characterize the particles themselves.
SIMI — works in concert with BMI and FMM by detecting light scattering, specifically high refractive index particles, including those protruding out of the membrane surface. Particles that scatter light indicate inorganic matter protruding out of the membrane such as glass or plastic. Particles that absorb light are indicative of metallic particles, such as residual Dynabeads™️, and absorbing oils that also protrude from the membrane.
A Diagnostic Case Study
Recently, Halo Labs scientists used the Aura+ analyzer to detect and characterize particles using BMI, FMM and SIMI, showing that the Aura+ was superior to other methods for distinguishing subvisible particles in cell therapies. This has become more important in therapeutic development in light of a recent FDA issuance of Form 483 concerning contamination of Kymriah® cell therapies with wood, cellulose, brass, and steel, thought to have entered through cryopreservation bags.
The researchers mixed Car-T cells with NIST particle standards, cellulose, and metallic particles. Car-T cells mixed with dynabeads were analyzed using SIMI mode on the Aura+. Together, BMI, FMM and SIMI allowed for total particle analysis in the complex particle cell mixture—the researchers found four particle subtypes—plastic, non-viable cells, viable cells and protein aggregates.
Contamination Control and Decontamination
Fortunately, good laboratory practices (GLP) can go a long way to manage the frequency and degree of culture contamination. It’s important to actively manage your cultures to reduce issues and keep accurate records for at least a month of any problem that arises, no matter how small.
Other tried-and-true methods of avoiding contamination include:
- Good aseptic techniques—providing a barrier between your cells and the environment.
- Reducing accidents—ensuring fewer spills, breakage or leaks.
- Keeping a clean lab—reduces biological, chemical and even physical particles from entering.
- Routine monitoring—keep records of any problem that arises.
- Avoid antibiotics—fend off resistant strains of bacteria
However, proper use of antiseptics (part of cleaning your lab) can be beneficial for fending off contamination.
Lab Standards, Quality Controls
Standards for laboratory practice fall under Good Laboratory Practice (GLP) methods, and quality controls are dictated by FDA standards, based on USP <788>.
USP <788>: specifically outlines the requirements for particle size limits (between 10 and 25 µm) and methods of particulate matter determination in injections for various dosage forms.
GLP: in the United States, these practices are under 21 CFR part 58. The European Community and key countries in Asia, Africa, the Americas, and the Middle East also follow these standards. According to the FDA:
GLP provides a framework for conducting well-controlled studies:
- Assures quality and integrity of the data
- Facilitates study reconstruction
- Provides overall accountability
- New compounds are first tested for safety via nonclinical studies
Contamination detection with Halo Aura
The Aura family of particle detection systems uses a combination of brightfield and fluorescent imaging, as well as light scattering to specifically identify and quantitate cell culture contaminants, using smaller samples than other methods and providing more accurate and precise readouts of your cell culture. The Aura family includes:
- Aura+; interdisciplinary biotherapeutics all in one system.
- Aura CL; for cell therapy, can identify cell aggregates and conduct cellular assays
- Aura GT; for gene therapy, can identify capsid aggregates, detect DNA leakage, conduct immunoassays
- Aura PTx; for immune (antibody) therapy, identifies polysorbate, and conduct immunoassays
References
Cell Culture: Growing Cells as Model Systems In Vitro https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7149418/#:~:text=Cell%20culture%20is%20one%20of,type%20cells%20and%20diseased%20cells.
FDA and GLP https://www.fda.gov/media/165993/download
USP <788> https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisionGeneralChapter788.pdf
Why particle analysis? https://www.halolabs.com/why-particle-analysis/
SIMI—How it works https://www.halolabs.com/simi-how-it-works/
Halo Labs Aura family brochure https://www.halolabs.com/resource/aura-family-brochure/
Virus Contaminations of Cell Cultures https://rdcu.be/dMCh0
DISCOVER THE AURA FAMILY
Read More ON this topic