The study of exosomes has gained substantial attention in recent years due to their significant potential in diagnostics, therapeutic applications, and as biomarkers for various diseases. Exosomes, which are extracellular vesicles (EVs) ranging from 30 to 150 nm when stable and in solution, are secreted by various cells and carry molecular signals like proteins, lipids, and RNAs to recipient cells. This function makes exosomes crucial in intercellular communication, disease progression, and immune modulation.
As a result, exosomes are now considered key players in the development of therapeutic drugs and disease detection methods. However, for researchers and pharmaceutical companies to fully harness the potential of exosomes, accurate quantification of these vesicles is essential. Whether in developing novel therapeutic strategies or detecting disease-specific biomarkers, the ability to accurately measure exosome concentration and cargo is pivotal.
This post explores the latest advancements in exosome quantification techniques, detailing how they impact pharmaceutical research and development, diagnostics, and drug delivery. We will highlight major tools used for exosome analysis, focusing on emerging technologies such as Halo Labs' Aura® system, which offers new levels of sensitivity and accuracy in exosome quantification.
What Are Exosomes?
Exosomes are small vesicles that are released from cells into extracellular space, playing a significant role in intercellular communication. They originate from the endosomal pathway and are formed by the inward budding of multivesicular bodies. When these bodies fuse with the cell membrane, exosomes are secreted into the extracellular environment. Due to their unique biogenesis, exosomes are equipped with biomolecules from their parent cells, including:
- Proteins: Membrane proteins, enzymes, and signaling molecules.
- Lipids: Cholesterol, phospholipids, and ceramides.
- Nucleic acids: RNA, including messenger RNA (mRNA) and microRNA (miRNA).
Exosomes can be isolated from various bodily fluids, such as blood, urine, cerebrospinal fluid, and saliva, making them accessible for clinical and research purposes. Their biological roles are diverse, ranging from immune modulation to facilitating cancer progression by transferring oncogenes between cells. Their ability to carry molecular signals makes them valuable as biomarkers for various diseases, including cancer, neurodegenerative disorders, and cardiovascular diseases.
Why Is Exosome Quantification Important?
Accurate exosome quantification is critical in ensuring consistency, reproducibility, and effectiveness in research and therapeutic development. Whether used for diagnostics or therapeutics, exosome concentration and cargo must be carefully measured to yield meaningful data.
- Therapeutic development: In drug development, exosomes are being explored as delivery systems for therapeutic molecules such as small interfering RNA (siRNA) and proteins. Precise quantification ensures accurate dosing, which is crucial for patient safety and treatment efficacy.
- Diagnostic applications: Exosomes are also used as biomarkers for early disease detection. For example, exosomes from cancer cells carry tumor-specific proteins and RNAs that can be detected in the blood, allowing for non-invasive cancer diagnosis. Accurate exosome quantification is essential in diagnostics to improve the sensitivity and specificity of these tests.
- Reproducibility: Accurate and consistent exosome quantification ensures that research data are reproducible across different experiments and laboratories. This is particularly important when translating research findings from preclinical studies to clinical applications.
Exosome Quantification Techniques
As the importance of exosomes in research and medicine continues to grow, so does the demand for more reliable and sensitive quantification methods. Currently, several established techniques are widely used to measure exosome concentration and characteristics. Each method offers unique advantages and limitations depending on the research application.
Nanoparticle Tracking Analysis (NTA)
Nanoparticle Tracking Analysis (NTA) is one of the most commonly used methods for exosome quantification. This technique allows researchers to measure both the size distribution and concentration of particles in a sample by tracking the movement of exosomes in a liquid suspension. Exosomes move via Brownian motion, and the velocity of their movement is used to calculate particle size.
Advantages of NTA:
- Simultaneous size and concentration measurement: NTA can provide detailed information on the size distribution of exosomes as well as their overall concentration in the sample.
- Minimal sample preparation: Unlike some other techniques, NTA typically requires less sample preparation, which reduces the risk of sample degradation and contamination.
- Real-time visualization: NTA offers researchers real-time data on particle movement, providing immediate feedback during experiments.
Limitations of NTA:
- Inability to distinguish between particle types: NTA does not differentiate between exosomes and other similarly sized particles, such as protein aggregates, unless the sample is thoroughly purified.
- Reproducibility issues: NTA can be sensitive to changes in experimental conditions, leading to variability in results. It also requires careful calibration and operator experience.
Despite its limitations, NTA remains one of the most commonly used methods for exosome quantification due to its ability to measure both size and concentration simultaneously. However, researchers must ensure that samples are well-prepared to avoid contamination from other particles.
Flow Cytometry
Flow cytometry is another widely used method for exosome detection and quantification. This technique involves labeling exosomes with fluorescent antibodies that bind to specific surface markers, such as CD63, CD9, and CD81. The labeled exosomes are then passed through a laser beam, and the resulting fluorescent signals are measured to quantify the number of exosomes in the sample.
Advantages of Flow Cytometry:
- High throughput: Flow cytometry allows for the rapid analysis of large numbers of exosomes, making it ideal for studies that require high-throughput data collection.
- Multiparametric analysis: Researchers can use different fluorescent labels to analyze multiple surface markers simultaneously, providing a detailed profile of exosome subpopulations.
- Specificity: By using antibodies that target exosome-specific markers, flow cytometry offers a higher degree of specificity than techniques like NTA.
Limitations of Flow Cytometry:
-
- Size detection limits: Traditional flow cytometers struggle to detect small particles like exosomes, leading to a lower sensitivity for particles below 100 nm.
- Complex sample preparation: The need for specific labeling can complicate sample preparation, potentially introducing variability or bias.
- Requires expertise: Set up and troubleshooting flow cytometers require significant experience and skills to prevent sample loss.
While flow cytometry is a powerful tool for exosome quantification, it is often used in conjunction with other techniques to overcome its sensitivity limitations.
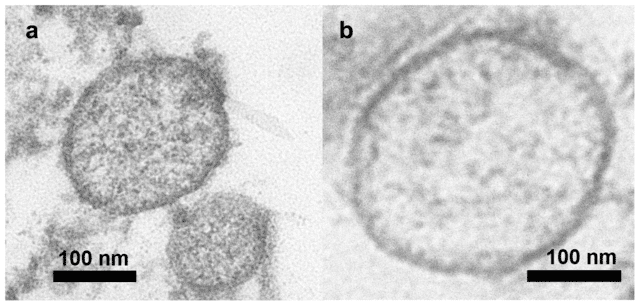
Transmission Electron Microscopy (TEM)
Transmission Electron Microscopy (TEM) is often used to visually confirm the presence of exosomes in a sample. TEM provides high-resolution images that allow researchers to assess the morphology and structural integrity of exosomes. While it is not primarily a quantification technique, TEM is valuable for verifying the quality of isolated exosomes.
Advantages of TEM:
- High-resolution imaging: TEM offers detailed images of exosome structure, allowing researchers to confirm the presence of intact exosomes.
- Morphological assessment: TEM can detect structural anomalies, such as damaged or aggregated exosomes, providing insights into the sample's quality.
Limitations of TEM:
- Labor-intensive: TEM requires extensive sample preparation and can be time-consuming, making it less practical for routine exosome quantification.
- Expensive: The equipment and expertise required for TEM are costly, limiting its use in many laboratories.
Emerging Techniques in Exosome Quantification
As exosome research advances, new technologies are being developed to address the limitations of traditional methods. One such emerging technology is Halo Labs’ Aura system, which combines high-throughput analysis with label-free particle detection.
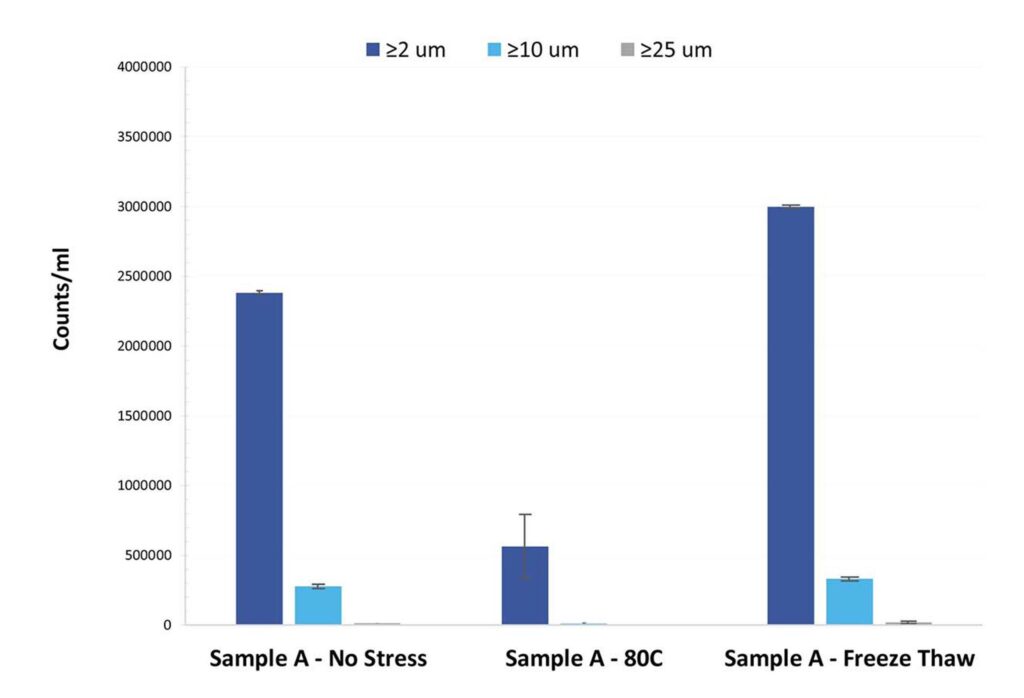
The Aura system is designed to provide more sensitive and accurate exosome quantification by detecting not only soluble, stable exosomes but also exosome aggregates. Exosome stability is critical in therapeutic applications, and detecting aggregates can help ensure that only functional, stable exosomes are used in downstream processes.
Advantages of Halo Labs’ Aura System:
- Ulra-Low sample volume: Unlike traditional techniques that require large sample volumes, the Aura system can quantify exosomes using as little as 5 μL of sample.
- Aggregate detection: Detecting exosome aggregates is essential for maintaining the stability and quality of exosome-based therapeutics. The Aura system excels in this area, providing more comprehensive data on sample quality.
- High-throughput capabilities: The Aura system is designed for high-throughput screening, making it ideal for large-scale studies or industrial applications where time and sample efficiency are critical.
For more information on how Halo Labs is advancing exosome quantification, visit their page on exosome detection solutions.

Exosome Analysis and Detection
While accurate quantification is critical, understanding the molecular composition of exosomes is equally important. Exosomes carry a range of biomolecules, including proteins, lipids, and nucleic acids, which can provide valuable insights into their biological function.
Exosome Markers for Detection
Exosome-specific surface markers are used to detect and characterize exosomes in biological samples. These markers are typically proteins, including:
- CD63, CD9, and CD81: Members of the tetraspanin family, these proteins are highly enriched on the surface of exosomes and are commonly used as markers in exosome research.
- Alix and TSG101: These proteins are involved in exosome biogenesis and are frequently used to confirm the presence of exosomes in a sample.
By targeting these specific markers, researchers can isolate and study exosomes from a wide variety of biological fluids, such as blood, urine, and saliva. The ability to detect exosomes in these fluids makes them attractive candidates for non-invasive diagnostics and biomarker discovery.
Immunoaffinity-Based Detection
One of the most specific and accurate methods for exosome detection is immunoaffinity-based techniques. These methods use antibodies that specifically bind to exosome surface proteins, such as tetraspanins. The antibodies capture the exosomes of interest, allowing for more precise quantification and analysis.
Advantages of Immunoaffinity-Based Detection:
- High specificity: By targeting exosome-specific markers, immunoaffinity methods can selectively isolate exosomes from complex biological samples.
- Sensitivity: Immunoaffinity techniques enhance the sensitivity of exosome detection by focusing only on vesicles expressing specific surface proteins.
Limitations:
- Limited to known markers: Immunoaffinity-based detection is restricted to exosomes with well-characterized markers. This may exclude exosome populations that do not express the targeted proteins.
- Sample preparation: Like flow cytometry, these methods require careful sample handling and preparation, which can introduce variability.
This technique is particularly useful in studies aiming to isolate exosomes from specific tissues or diseases, such as cancer or neurodegenerative disorders, where exosome subtypes play key roles in disease progression.
Challenges and Limitations in Exosome Quantification
While significant advancements have been made in exosome quantification, several challenges and limitations remain, particularly in sample preparation and technical precision.
Sample Preparation Challenges
One of the biggest obstacles in exosome research is ensuring the purity of exosome samples. Biological samples often contain a mix of extracellular vesicles, protein aggregates, and other particles that can interfere with exosome quantification. If these contaminants are not effectively removed, they can skew results and lead to inaccurate measurements.
Common methods for exosome isolation, such as ultracentrifugation and size-exclusion chromatography, are frequently used to purify samples. However, these methods are time-consuming and may not completely eliminate contaminants. Furthermore, small sample volumes can complicate the isolation process.
Best Practices in Sample Preparation:
- Use of optimized isolation protocols: Using well-validated protocols and kits for exosome isolation can help reduce contamination and improve the accuracy of quantification.
- Multiple rounds of purification: In some cases, researchers may need to use multiple isolation techniques, such as combining ultracentrifugation with immunoaffinity capture, to enhance sample purity.
Technical Limitations
Several technical limitations also hinder accurate exosome quantification, particularly in the reproducibility and sensitivity of current techniques. For example, flow cytometry and NTA struggle to provide consistent results across different laboratories due to variations in sample handling, instrument calibration, and operator expertise.
Additionally, these traditional methods often have difficulty detecting smaller or more heterogeneous exosome populations, especially when the exosome concentration is low.
How the Aura System Overcomes Technical Challenges:
The Aura system by Halo Labs addresses many of these technical limitations. Unlike traditional methods, the Aura system requires minimal sample volumes and provides high sensitivity, making it ideal for detecting low-abundance exosomes in complex samples. Moreover, its ability to detect exosome aggregates adds another layer of analysis, ensuring that researchers are working with stable and functional exosomes.
For more details on how Halo Labs' Aura systems can help overcome these technical limitations, visit their exosome solutions page.
Applications of Exosome Quantification
Accurate exosome quantification has a wide range of applications in both research and clinical settings. From diagnostics to therapeutics, the ability to measure exosome concentration and cargo can significantly advance the development of new treatments and disease monitoring tools.
Exosome Quantification in Diagnostics
One of the most promising applications of exosome quantification is in the field of diagnostics. Exosomes carry molecular signatures from their parent cells, making them valuable tools for detecting disease biomarkers. For instance, exosomes isolated from cancer cells contain oncogenic proteins and RNAs that can be detected in the blood, allowing for the early diagnosis of tumors.
Exosomes are particularly useful in the diagnosis of diseases such as:
- Cancer: Tumor-derived exosomes carry specific proteins and RNAs that can be used as biomarkers for early cancer detection and monitoring.
- Neurodegenerative diseases: Exosomes in the cerebrospinal fluid of patients with Alzheimer's disease or Parkinson's disease carry disease-specific proteins that may provide insights into disease progression.
- Cardiovascular diseases: Exosomes released by injured heart tissue carry molecular signals that can be detected in blood samples, offering non-invasive methods for diagnosing heart conditions.
Accurate quantification of exosomes in these fluids is critical for developing sensitive diagnostic tests that can detect diseases at early stages, when they are most treatable.
Advantages of Exosome-Based Diagnostics:
- Non-invasive sampling: Exosomes can be isolated from easily accessible body fluids like blood and urine, reducing the need for invasive biopsies.
- Real-time monitoring: Because exosomes are constantly being released by cells, they can be used to monitor disease progression or treatment response in real-time.
Exosome Quantification in Therapeutic Development
In the field of therapeutics, exosomes are being explored as potential drug delivery vehicles. Due to their natural ability to carry and deliver cargo to specific cells, exosomes are well-suited for the delivery of therapeutic molecules, such as small interfering RNA (siRNA), proteins, and drugs.
Accurate quantification of exosomes is essential in therapeutic development to ensure that the appropriate dose is delivered. Exosome-based drug delivery offers several advantages over traditional drug delivery systems, including:
- Biocompatibility: Since exosomes are naturally occurring, they are less likely to be immunogenic or toxic compared to synthetic nanoparticles.
- Target specificity: Exosomes can be engineered to carry therapeutic cargo to specific cell types or tissues, enhancing the effectiveness of the treatment.
Exosomes are also being investigated for use in gene therapy. By loading exosomes with genetic material, such as siRNA or CRISPR components, researchers can selectively edit genes in target cells. This precision in gene delivery makes exosomes a powerful tool in the development of personalized medicine.
Future Directions in Exosome Quantification
The field of exosome quantification is rapidly evolving, with new techniques and tools constantly being developed to improve sensitivity, specificity, and throughput. As researchers continue to explore the therapeutic and diagnostic potential of exosomes, the demand for more accurate and scalable quantification methods will only grow.
One of the most exciting trends in exosome research is the integration of machine learning and artificial intelligence (AI) in data analysis. By leveraging AI algorithms, researchers can analyze large datasets from exosome studies, identifying patterns that may not be visible to the human eye. These insights could lead to the discovery of new exosome biomarkers or therapeutic targets.
Additionally, advancements in microfluidic technologies are expected to revolutionize exosome isolation and quantification. Microfluidic devices can precisely manipulate small volumes of fluid, allowing for the rapid and efficient isolation of exosomes from biological samples. As these technologies become more accessible, they will likely play a central role in future exosome research.
Conclusion
Exosome quantification is a rapidly advancing field that plays a critical role in both diagnostic and therapeutic applications. The accurate measurement of exosome concentration, size, and cargo is essential for ensuring reproducibility, consistency, and safety in research and drug development. While traditional methods like Nanoparticle Tracking Analysis (NTA) and flow cytometry remain popular, emerging technologies such as Halo Labs’ Aura system offer new levels of sensitivity and efficiency, particularly in detecting exosome aggregates and requiring minimal sample volume. Developers should be conscientious of biophysical stability of their exosome therapeutics to ensure patient safety and drug efficacy.
Exosomes hold immense potential in modern medicine, offering non-invasive diagnostic tools and highly specific drug delivery systems. However, realizing this potential requires the adoption of advanced quantification techniques and tools. By leveraging the latest advancements, researchers and pharmaceutical companies can push the boundaries of exosome-based research and develop new therapies that will transform healthcare.
As the field continues to evolve, innovations in exosome quantification will undoubtedly lead to further breakthroughs in disease detection, personalized medicine, and targeted therapies. Researchers and pharmaceutical developers who embrace these technologies will be well-positioned to lead the next wave of advancements in biotherapeutics.
For more information on cutting-edge exosome detection and quantification solutions, visit Halo Labs and learn how the Aura system can help optimize your research and development efforts.
Sources
- Zhang Y, et al. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2.
- Lai JL , et al. Adv Sci (Weinh). 2022;9(15):2103222. doi: 10.1002/advs.202103222.
DISCOVER THE AURA FAMILY
Read More ON this topic