Pharmaceutical companies go to great lengths to ensure the stability of their products. They test for it, they plan for it, and they design around it. Why? Because stability is essential to the therapeutic success of a product. Without it, maintaining efficacy and safety would be impossible. Proteins are particularly susceptible to aggregation, which can lead to immunological effects that are detrimental to the patient.
As a result, regulatory agencies expect drug developers to characterize protein aggregates and control their presence in injectable formulations – which shows just how important it is to have effective tools that can monitor particle levels.1
Traditional Analytical Techniques: What You’re Not Seeing Can Hurt You
Several analytical techniques are currently used to measure the size, shape, and distribution of particles in a sample. However, with current subvisible analysis techniques, companies can only measure a tiny fraction of the aggregates present in formulations, leaving them in the dark about potential risks.
- Microscopy: As the most fundamental analysis technique for characterizing particles, microscopy provides detailed information on size and morphology. While it is a low-throughput method, it offers the advantage of being a direct counting method that allows for the analysis of each sample in its entirety.1 Still, microscopy is labor intensive and requires trained analysts.
- Light Obscuration (LO): Light obscuration is an optical technique that measures the loss of light intensity that occurs as particles flow through a beam of light.1 However, LO methods cannot accurately count particles in high-viscosity formulations and cannot assess particle morphological information. This method also does not provide the same level of detail as microscopy, as particles that are transparent and non-spherical often do not effectively obscure light and are thus undercounted in LO.
- Micro Flow Imaging (MFI): A commonly used method is micro flow imaging, also referred to as imaging microscopy (FIM) or dynamic imaging analysis (DIA). This method of dynamic imaging particle analysis captures images of particles as they flow through a cell and then uses images to calculate size, shape, and count based on a pixel-by-pixel analysis.1 While this technique provides valuable information, there are drawbacks. For example, the images can be difficult to interpret, and the process is time-consuming. And because this method is fluidics-based, it requires higher sample volume and user expertise. Pairing that requirement with the risk of clogging, leaking, or instrument failure is a big fear.
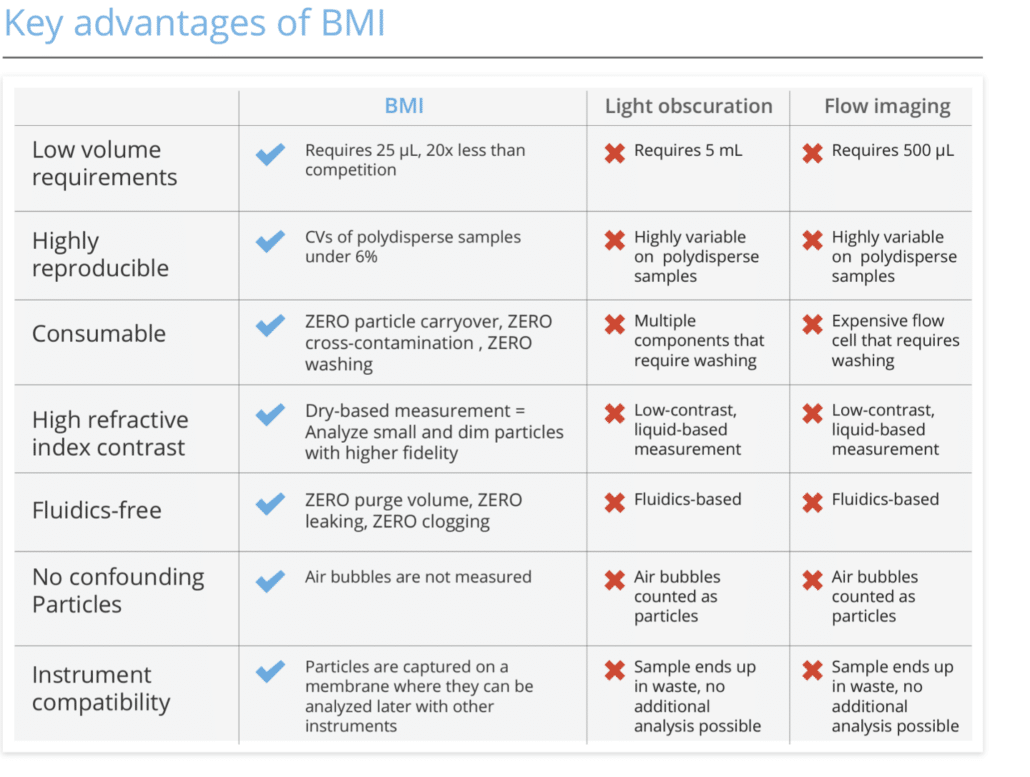
Get the Whole Picture with BMI: High Contrast Particle Imaging for Visible and Subvisible Analysis
To help overcome some of these challenges with today’s current methods, Halo Labs has developed a new particle analysis technique called Background Membrane Imaging (BMI). BMI uses a light microscope to image the surface of a membrane and then applies advanced image processing algorithms to detect and quantify the particles.
BMI reinvents membrane imaging with modern robotics, image processing, and novel optics in a 96-well filter plate format that works just like a plate reader. This technique has several advantages over traditional methods, including greater sensitivity and the ability to image large areas of the membrane at once. In addition, BMI does not require the use of expensive equipment or specialized training.
BMI uses sophisticated image-processing techniques to analyze images and acquire particle data. The key is first to take a background image of the membrane. After samples are filtered through, and particles are captured, the same membrane is re-imaged, this time with particles on the surface. The background image is precisely aligned with the sample image and then subtracted on a pixel-by-pixel basis to eliminate the background texture and reveal particles. Contrast is 10x greater than measurements done in liquid, sizes are calibrated with an electron microscope, and analysis is fully automated.
Recent Studies Demonstrate Effectiveness of BMI
In a recent study, Halo Lab’s Aura® platform (formerly known as HORIZON) was optimized and compared to LO, the MFI™ 5200 Series by ProteinSimple, and the FlowCam® 8000 Series by Fluid Imaging Technologies. The study compared precision, linearity, subvisible particle (SVP) concentration, and morphological output of BMI compared to the other three techniques. BMI was shown to be a useful tool in measuring protein aggregates in a high-throughput manner. The authors showed that it is a simple, fast method and can produce similar or better precision and sensitivity to current dynamic flow imaging techniques, eliminating the need for large sample volumes and long analysis time.1
In a second study, the goal was to evaluate BMI for its use in the analysis of SVPs and to compare it to DIA methods for pharmaceutically relevant drugs. The authors wrote, “The team demonstrated BMI to be a suitable orthogonal method for the characterization of subvisible particulate matter in biopharmaceutical products. Especially, simplicity of sample processing, high achievable throughput, use of disposable consumables, and low required sample volume can make BMI an attractive alternative or complement to particle analysis by DIA. In particular, BMI might be a highly valuable tool for high-throughput screenings, for example, in formulation development, where various formulations with different excipients and thus disparate properties (e.g., refractive index) need to be compared.”2
Based on their findings, the authors concluded: “We suggest an adaptation of the Aura hardware to enable imaging of complete wells, therefore, enabling accurate particle counting.”2
The results of these studies demonstrate that BMI can be successfully applied to characterize subvisible particulate matter in biopharmaceutical products. This technology offers a simple and efficient approach for the characterization of these particles, which is valuable for ensuring product quality and safety.
With Halo Lab’s Aura PTx, which utilizes BMI as its primary analytical technique, there’s a better way to perform particle analysis. Aura PTx makes it easy to quickly identify and count aggregates that form due to degraded polysorbate in your formulation. And with two-channel fluorescence, you can determine if aggregation in your protein therapy is caused by proteins or polysorbates in your sample – at the same time.
To learn more about background membrane imaging and how Aura PTx can get you to your best formulation faster, visit: www.halolabs.com/aura-ptx-aggregate-analysis.