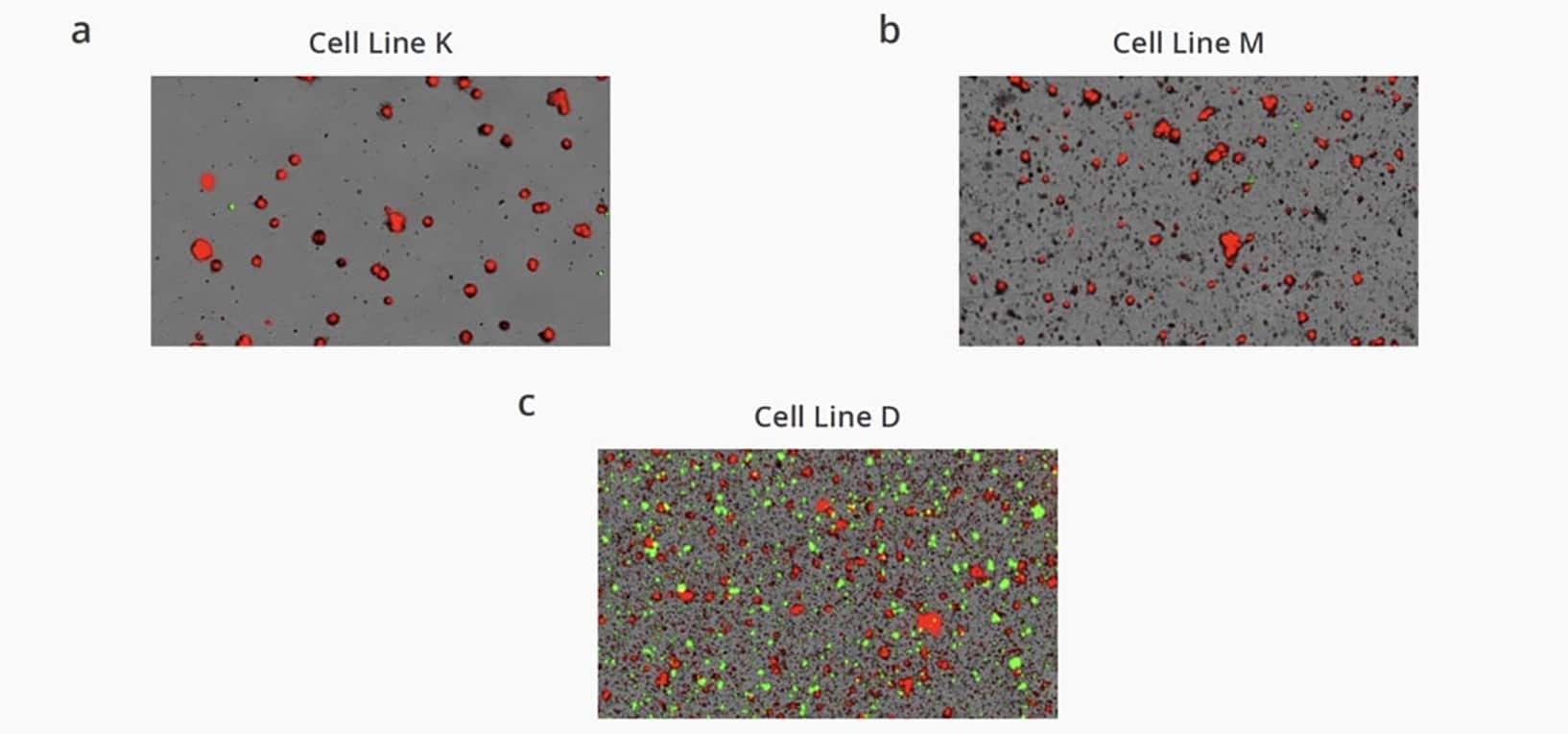
Low Sample Antibody Stability Results
Thanks to their inherent scalability, CHOs transformed modern therapeutic antibody production, producing proteins with mammalian post-translational modifications, high titers, and a long regulatory record. While cell lines are often tested during development for their capacity to produce titers, stability testing is a crucial part of this development: evaluating genotypic, phenotypic and functional stability of the cell line over multiple replication cycles or passages. Halo Labs’ Aura immunoassays with particle characterization bridge the gap between cell line development and therapy developability, prescreening for antibody ability once the mAbs are secreted from CHO cells.
Why Use Aura in Your Cell Line Development Workflow?
Aura instruments deliver details on particle images, size, count, and identity, advancing your therapeutic products’ quality, stability, and purity.
- Find subvisible particles that plague biologics and can interfere with antibody stability
- Start measuring particles and antibody stability as early as the cell line development stage and through product development and beyond, with very small sample sizes and with unfiltered CHO cell samples
- Quickly visualize and differentiate between high-, mid-, and low levels of antibody aggregation, and quickly distinguish between CHO cells and subvisible antibody aggregates
- Get high-level high-throughput insights in minutes (96 samples in 96 minutes)
- Rapidly find manufacturable molecules in the developability stage
- Fluorescence Membrane Microscopy (FMM) antibody labeling adapts to your existing fluorescent workflows
- Rank CHO cell lines by mAb stability
- Distinguish between proteins and degraded polysorbate particles
- Compatible with USP 788, and 21 CFR Part 11 software available
All the Data You Need to Characterize Antibodies
The Aura+ and Aura PTx can quickly and accurately characterize secreted antibody stability during CHO cell line development in unfiltered cell line samples. These systems deliver low volume, high-throughput particle imaging, counting, sizing and identification. They can also rank antibodies according to this stability data, showing a new, valuable way to decide on drug candidates that goes beyond titers.
Improve Your Efficiency in Analyzing Complex Cellular and Protein Samples
Aura+ can rapidly assess different formulation strategies for the amount of protein and potential interference from particles, by imaging and characterizing the types and counts of proteins and non-protein aggregate in each sample. For the first time, Aura can distinguish ubiquitous polysorbate degradation molecules from other protein aggregates. These aggregates can interfere with protein stability, cause immunogenic reactions, reduce efficacy, and shorten the drug’s shelf life.
Featured products
Frequently Asked Questions
Cell lines, such as CHO, are selected for their ability to produce proteins with mammalian post-translational modifications. Development using the Aura system can analyze the characteristics of the protein and its accompanying particles for optimal protein production and stability.
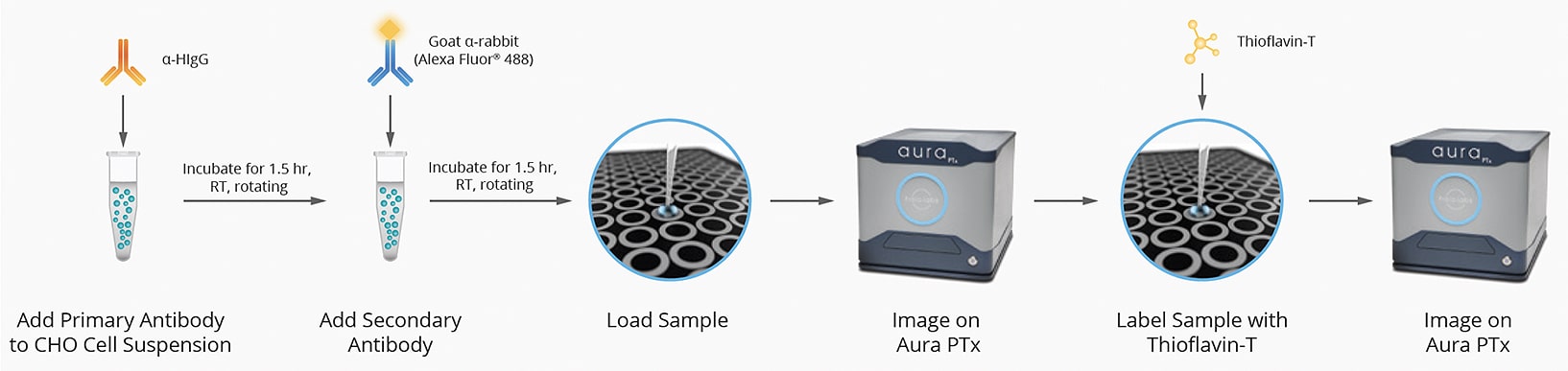
Primary and secondary antibodies with compatible fluorophores are added to CHO cell suspensions, and then samples are loaded and imaged on the Aura PTx (Aura+ can perform this function, too). Samples are then compared for stability by looking at concentrations of proteins and non-protein aggregates.
Cell line growth begins with the cell line development phase, continues through developability analysis, then formulation, followed by manufacturing and release, and post-market analysis. Aura systems can determine the best therapies during any of these stages.
Chinese Hamster Ovary cells are the originators of nearly all cell lines for antibody and other biotherapeutics, and until recently, developers have been free (and at the same time, pressured) to focus on creating the highest titers possible. But new, complex biological demands, such as bispecifics and other antibodies that have low expressions, dramatically higher concentration requirements for injectables, and larger market demands have required a pivot to other methods. The Aura system can find the optimal cell line development that balances high titers and protein stability with today's therapeutic market demands as well as addressing the challenges to development.