Do You Know What's Really in Your Sample?
Traditional fluidic imaging cytometry notoriously falls short while distinguishing between clusters of cells and other non-cellular material, leading to compromised results.This is only exacerbated by the difficulty of avoiding clogging and cross-contamination, further decreasing effectiveness.
It's evident that new technologies need to be developed to help bridge this gap and make accurate cell identification more efficient.
With Aura CL, you now have a solution.
Why Use Aura to Identify Cellular and Non-Cellular Particles?
- Definitively ID subvisible particles using fluorescent cell and other particle specific stains
- Obtain detailed information on particles that other methods can’t deliver, including size, morphology, count, and distribution
- Small to large cellular aggregate characterization in highly heterogeneous solutions
- 100x higher throughput compared to standard flow imaging and cytometry techniques for testing lots of formulations, conditions, and lot releases
- Fluidics-free rapid analysis time
- Evaluate a wide range of particle sizes, measuring 1 μm to 5 mm with high reproducibility
- Maintain compliance with the option for 21 CFR Part 11 software
Accurately Distinguish Between Cellular and Non-Cellular Particles
Aura CL stands out from other fluidic imaging cytometry techniques with its Fluorescent Membrane Microscopy (FMM) technology analyzing cells in the solid phase. For the first time, you can tell if your aggregate is cellular or non-cellular in your cell therapy, enabling you to develop a better understanding of quality attributes.
Aura CL delivers precise cellular particle count and characterization for superior quality assessment in the production process—so you get only the best therapeutic results.
Plus, state-of-the art features like fluidics-free prevent clogging issues seen in other methodologies. It enables high-throughput, high refractive index contrast, and 100% measurement efficiency for suspended cells, protein, and viral aggregates found in cell and gene therapies.
Make Better Decisions About Your Therapeutics
With Aura CL’s powerful, low-volume imaging capabilities, you can easily label cells in your samples with DNA-specific dyes and detect protein aggregates using Thioflavin T (ThT). Plus get unparalleled insight by combining this data with the distinct morphology information obtained via Backgrounded Membrane Imaging (BMI) and Side Illumination Membrane Imaging (SIMI) — all to reveal an unbiased view into every particle.
This comprehensive overview makes it easier than ever to make better decisions more quickly.
Accurately Monitor Cell Behavior
With Aura CL, you can easily determine if your therapeutic formulation or storage is the cause of cell aggregation.
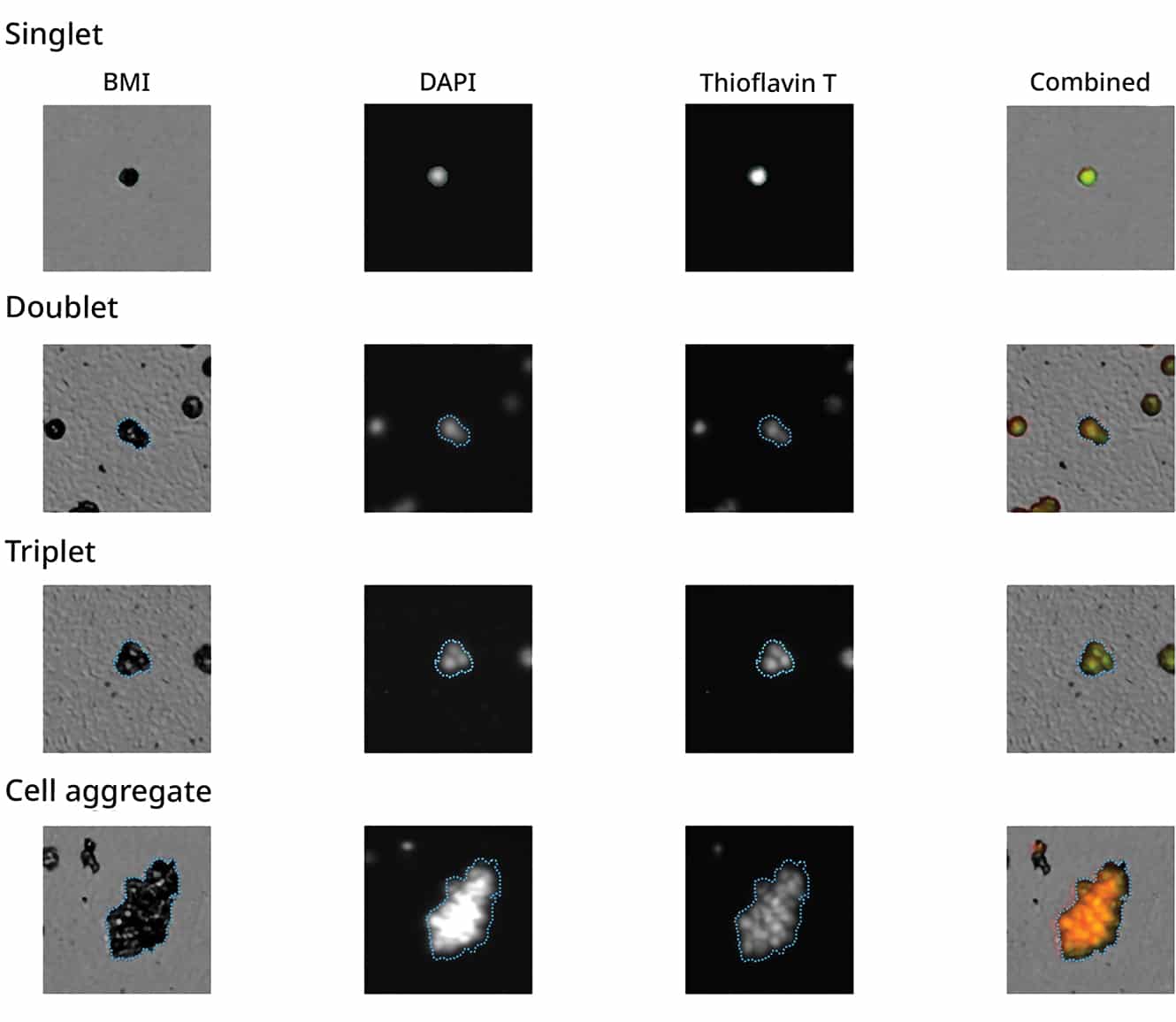
Utilizing either DAPI or Hoechst staining to label nuclei and performing a comparison with BMI imaging gives an in-depth overview on whether singlets, doublets, triplets and beyond are present within your cellular population.
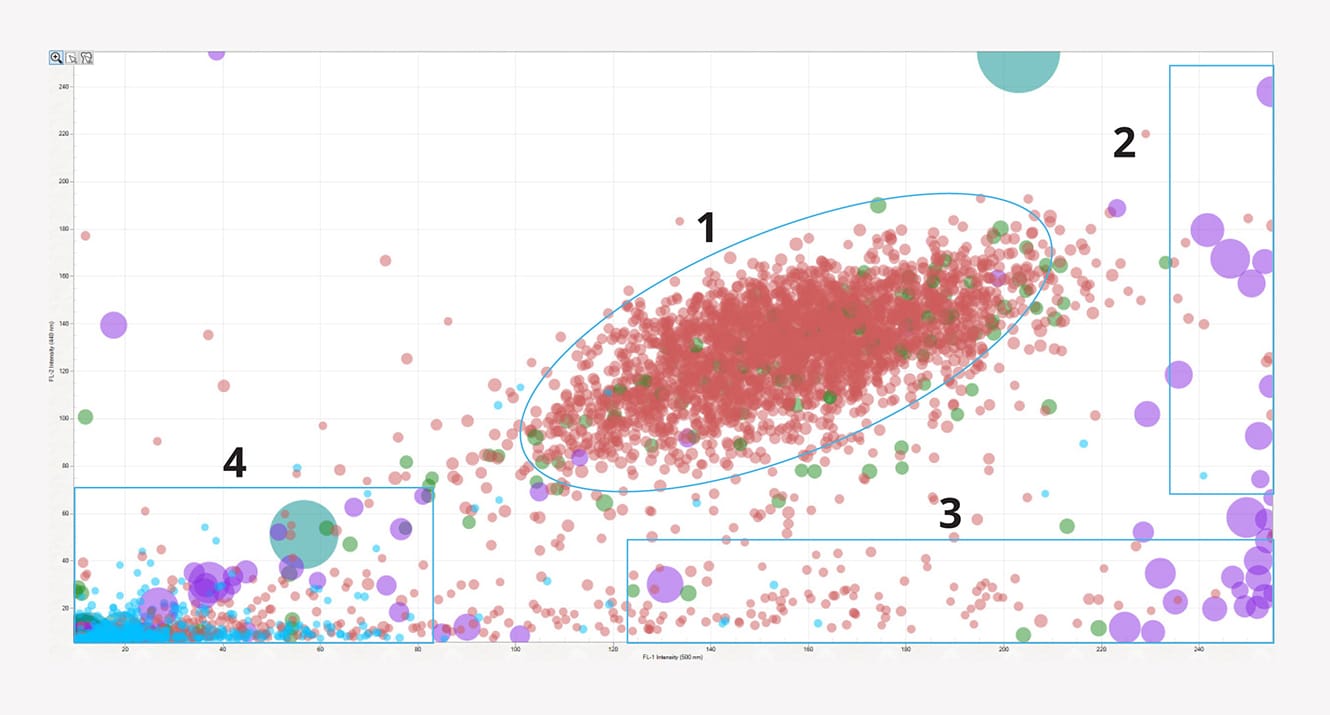
Particles can be identified through their fluorescent properties and overall area. See an example from each quadrant with singlets and doublets presenting a dual positive identification for DNA and protein based on the intensity of FL1 and FL2 measurements. Non-biological aggregates show negative for DNA and protein, while protein aggregates show particles positive for protein only.

Particle Vue Software
Get particle analysis answers in just a few clicks with flexible, easy-to-use Particle Vue Software.
Featured products
Frequently Asked Questions
All cells share certain fundamental characteristics, some of these including:
- Plasma Membrane: Cells are surrounded by a plasma membrane that separates the cell from its environment and regulates the passage of molecules in and out of the cell.
- Genetic Material: Cells contain genetic material, typically DNA (deoxyribonucleic acid) in the form of chromosomes, which carries the instructions for cell function and heredity.
- Cytoplasm: The cytoplasm is the gel-like substance inside the cell that contains various organelles, such as mitochondria, endoplasmic reticulum, and ribosomes, involved in cellular processes.
- Metabolism: Cells carry out metabolic processes to obtain energy and synthesize biomolecules necessary for cell growth, maintenance, and function.
- Reproduction: Cells have the ability to reproduce and divide to produce new cells through processes such as mitosis or meiosis.
These fundamental characteristics are essential for the survival and function of all cells, regardless of their type or function within an organism.
Cell characterization assesses various properties and attributes of cells to understand their identity, behavior, and function. Common techniques used for cell characterization include:
- Morphological Analysis: Observing cell shape, size, and structure using microscopy or imaging techniques.
- Cell Proliferation and Viability Assays: Assessing cell growth, proliferation, and viability using techniques such as cell counting, metabolic assays, or flow cytometry.
- Cell Surface Marker Analysis: Identifying specific cell surface markers or antigens using techniques such as flow cytometry or immunofluorescence staining.
- Genetic Analysis: Analyzing gene expression profiles, mutations, or genetic modifications using techniques such as PCR (polymerase chain reaction) or gene sequencing.
- Functional Assays: Evaluating cellular functions such as migration, differentiation, or cytokine secretion using specialized functional assays.
Cell characterization provides valuable insights into cell identity, behavior, and function, aiding in research, drug development, and clinical applications.
Cell line testing involves assessing the characteristics, purity, and stability of cell lines used in research, drug development, or manufacturing processes. This includes verifying the identity of the cell line, ensuring absence of contamination, and monitoring genetic stability over time. Cell line testing is essential to maintain reproducibility, reliability, and safety in experimental and therapeutic applications involving cell lines.