Identifying Stable Biologic Candidates During CLD: Leveraging the Power of Immunoassays
June 12, 2023
CHO cell line development (CLD) has revolutionized modern therapeutic antibody production due to their scalability benefits, ability to produce proteins with the necessary mammalian posttranslational modifications, and large yield protein titers.1
To meet the exponentially growing demand, CHO cell line developers have focused their attention on maximizing titer. However, newer and more complex biologic modalities such as bispecifics and other types of antibodies that display low expression yield, dramatically higher concentration requirements of injectables, and significant market demands have pushed CHO cell lines to their limit.1
While progress has been made, little has been done to characterize the physical stability of secreted antibodies from their inception during CHO cell line development. Scientists have been pushed to stabilize biologics that were not designed with aggregation in mind from the ground up, often with limited success.
Cell line stability testing is a crucial aspect of cell culture research and development. This process involves evaluating the genetic, phenotypic, and functional stability of a particular cell line over multiple passages or replication cycles. It is performed to ensure that the cultured cells exhibit consistent characteristics and behavior, which is essential for reliable and reproducible experimental results. Additionally, cell line stability testing helps identify potential issues such as genetic drift, contamination, or phenotypic changes that may occur during long-term cultivation. By monitoring and verifying the stability of cell lines, scientists and researchers can confidently use them in various applications, including drug discovery, biotechnology, and regenerative medicine.
Faster, More Accurate Antibody Stability Pre-Screening
Cell line stability assessment is a critical step in evaluating the long-term viability and consistency of a particular cellular model. Halo Labs' Aura® immunoassays with particle characterization bridge the gap between cell line development and developability to enable pre-screening for antibody stability once mAbs are secreted from CHO cells. Analyzing biologically complex cellular and protein samples present in CLD to characterize secreted protein stability is now faster and more accurate. Our Aura systems provide an easier way for protein therapeutic developers to rank and select CHO cell line winners.
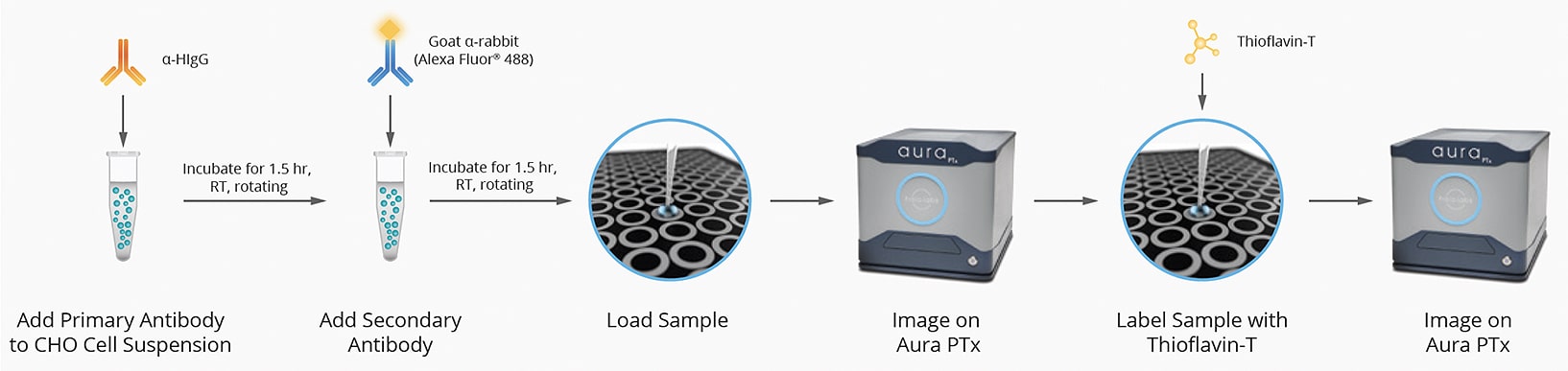
To demonstrate their effectiveness, we conducted a study in which we used an established antibody labeling protocol (Figure 1) that required only a 40 µL sample to characterize protein titer and rank cell lines according to the physical stability of the secreted antibody.
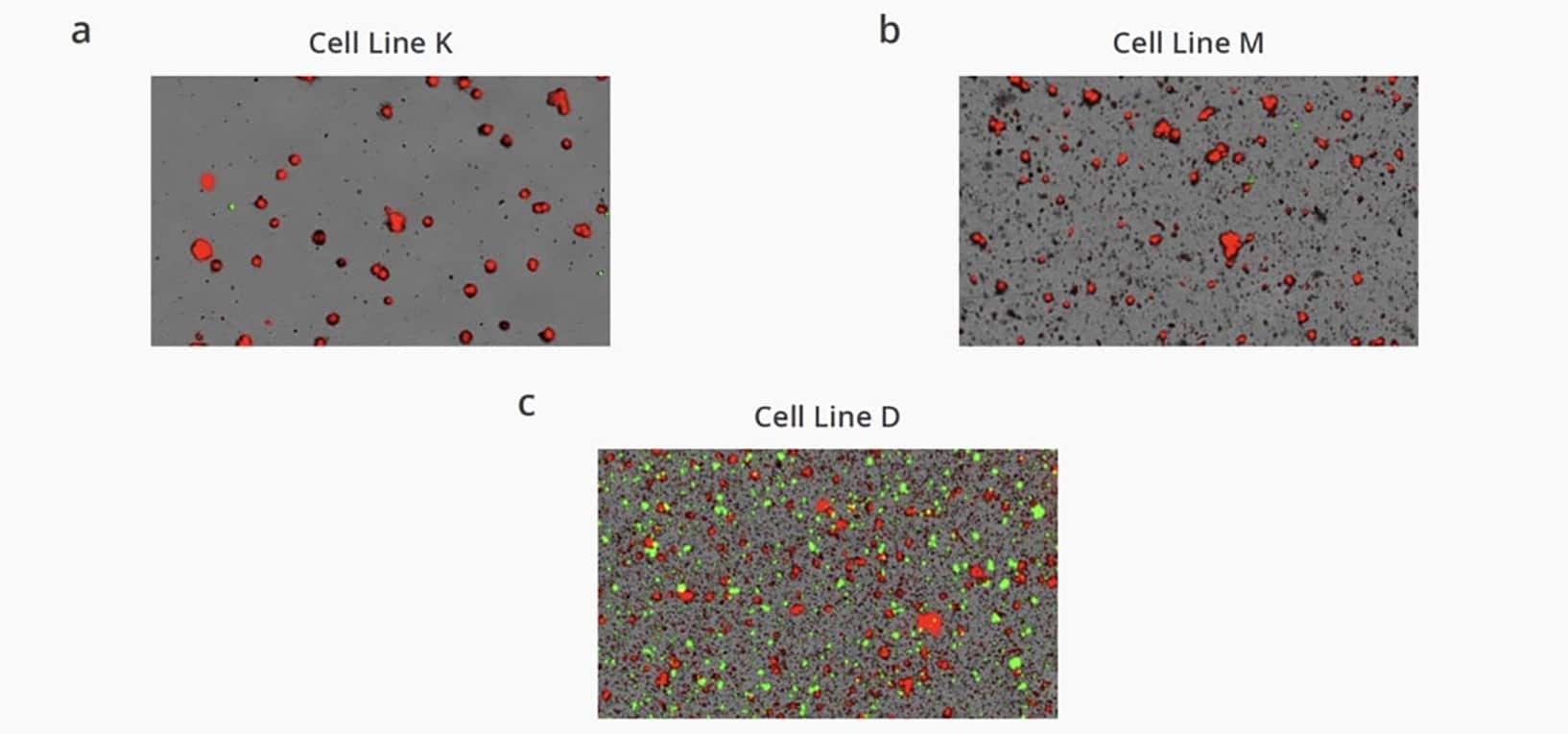
We imaged and analyzed various antibody secreting CHO cell lines using Aura PTx. Images were acquired in brightfield, FL1 Thioflavin T (ThT, red), and FL2 human IgG (HIgG, green) channels. These images allowed us to quickly grasp the secreted antibody stability. As seen in Figure 2, Cell Line K was the most stable with almost zero antibody aggregation, Cell Line M was moderately stable, and Cell Line D was the least stable with several orders of aggregates above control observed. The results demonstrate how Aura PTx can quickly visualize and easily differentiate between high-, mid-, and low-levels of antibody aggregation using the Aura immunoassay, and quickly distinguish if the particles measured are CHO cells or subvisible antibody aggregates.
Cell Line Rankings Based on mAb Stability
Aura PTx with Particle Vue software performs high throughput characterization and identification of subvisible aggregates across an entire experiment, enabling the ranking of samples based on stability. The 15 cell line candidates were ranked for commercialization according to antibody stability based on the total area of particle fluorescence observed in the FL2 HIgG antibody channel (green). The immunoassay results display drastic differences between various CHO cell lines depending on the stability of the secreted antibodies.
With Aura immunoassays, stability testing has never been easier. You can now screen for stability of antibodies in CHO cells quickly and efficiently with only 40 μL per well. This low-volume method provides positive results within 2 minutes or less, resulting in painless analytical measurements that don't require entire flasks of cells. Our Particle Vue software automatically characterizes and quantifies subvisible antibody aggregates visually, giving you a high-level ranking on each sample. Stored images deliver granular insights into the types of physical instabilities found in biologics for deeper understanding.
And it's not just about our immunoassay method. Aura systems enable quick characterization of unfiltered cell lines samples through low-volume, high-throughput imaging, counting, sizing and identification. Analyzing biologically complex cellular and protein samples present in CLD to characterize secreted protein stability has never been faster or more accurate.
To learn more about how you can leverage the power of immunoassays during cell line development, download our app note: App Note 18: Identify Stable Biologic Candidates During Cell Line Development