blog
Identifying Stable Biologic Candidates During CLD: Leveraging the Power of Immunoassays
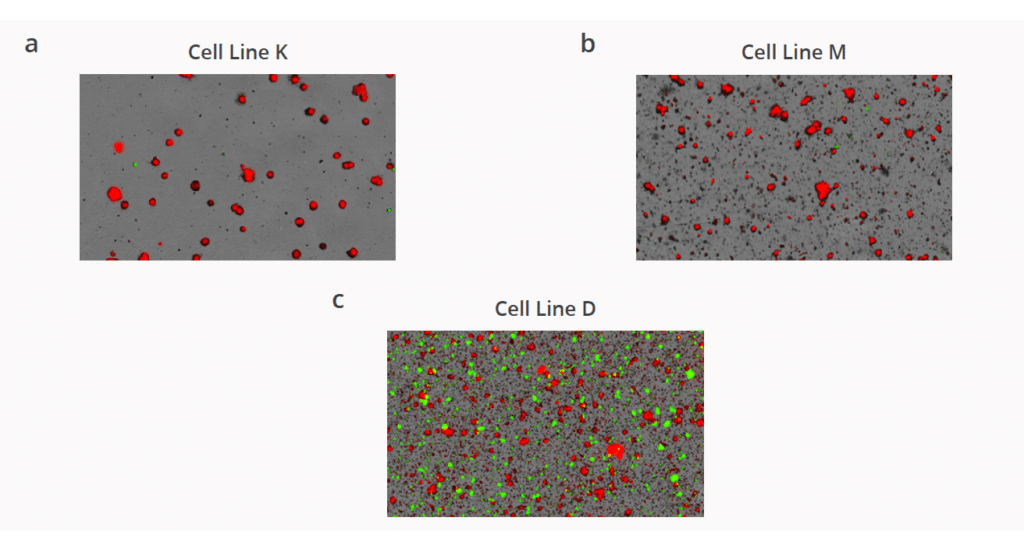
CHO cell line development (CLD) has revolutionized modern therapeutic antibody production due to their scalability benefits, ability to produce proteins…
Read More