The Smarter Way to Develop Cell Therapy Products
Cell therapy development has long been hindered by traditional techniques, which are limited due to their low-throughput and inability to distinguish cells from subvisible counterparts and non-cell contaminants.
Aura CL has broken that barrier, becoming the first system to quantify cell aggregates and identify cell versus non-cell contaminants—all with minimal sample material.
Now smarter insights are available faster than ever before thanks to this breakthrough.
Characterize CAR-T Cell Products with Cutting-Edge Technology
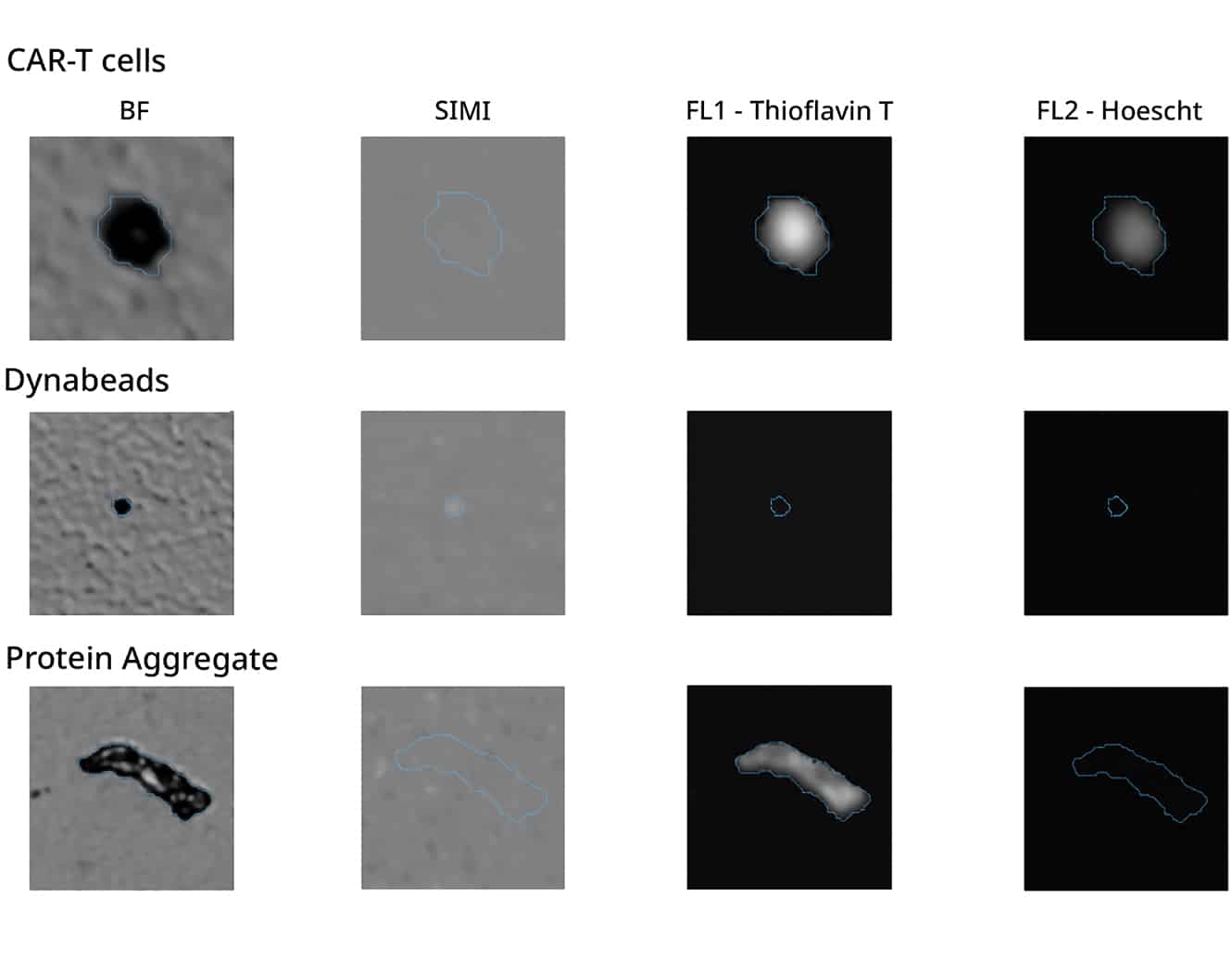
Leverage cutting-edge technology to get the most out of your cell therapy development with Halo Labs’ powerful duo: Backgrounded Membrane Imaging (BMI), Fluorescence Membrane Microscopy (FMM), and Side Illumination Membrane Imaging (SIMI).
Just 5 µL of material is all you need to quickly and accurately characterize CAR-T cell therapy products.
And the best part? When you pair this combo with Particle Vue Software's Expression Engine tool, you can obtain high-level insights about the cells across entire experiment datasets.
Measure Cell Viability and Product Purity
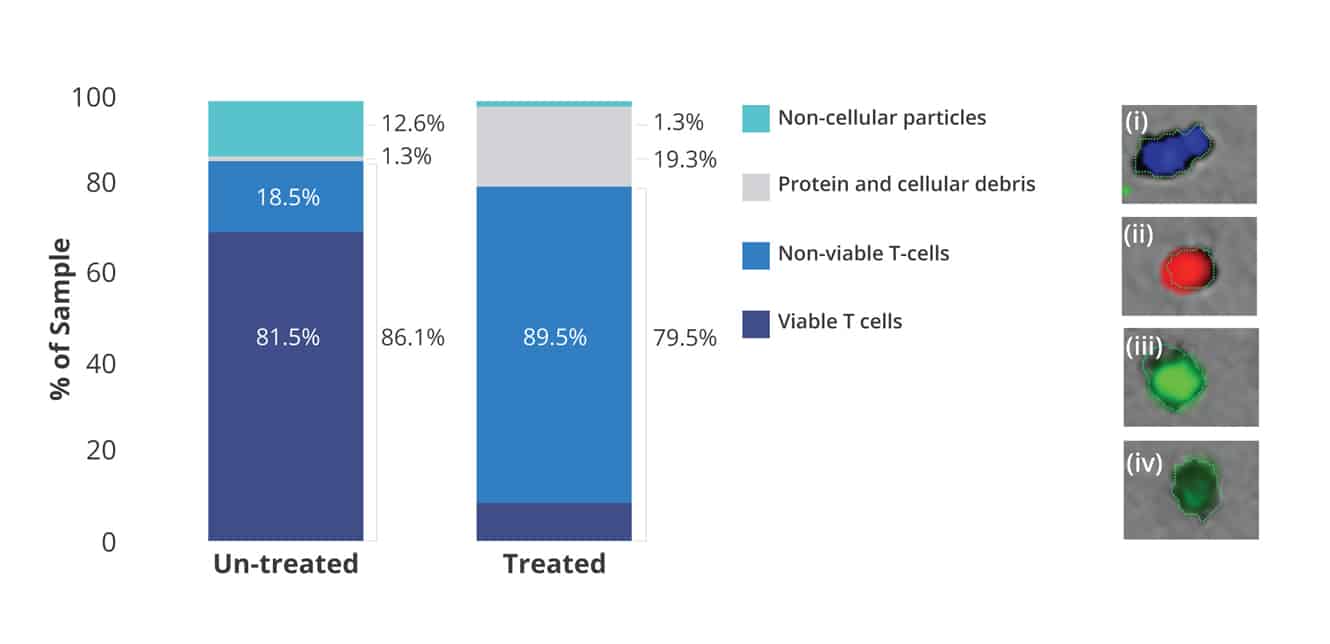
With Aura CL, researchers now have access to a high-throughput cell viability assay that rivals the accuracy of flow cytometry and hemocytometry assays.
In a 96-well format, you can now evaluate cell viability at high throughput speeds with low volumetric consumption, unlike traditional techniques.
What’s more, concentrated samples can be processed without risk of clogging, and every cell or particle measurement is visually confirmed for an added level of reassurance.
All this makes it possible for characterization of cell viability in tandem with product purity measurements.
Get a Closer Look at Dynabeads with High Magnification Imaging
As a cell therapy developer, you want to ensure your products are free of contaminants that could interfere with efficacy and safety.
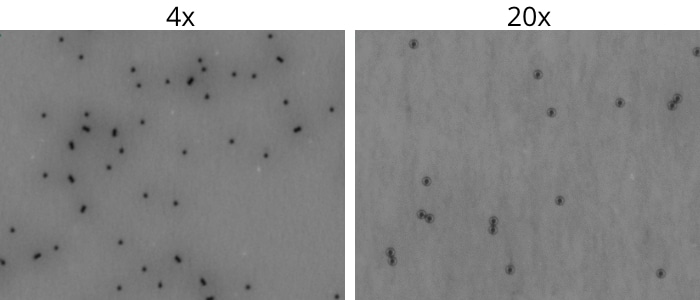
With Aura CL, we offer the first and only solution designed to quickly and accurately find and count residual Dynabeads™️ in concentrated cell therapy products.
This one-of-a-kind technology offers an unbeatable combination of brightfield imaging and SIMI to easily identify and quantify low levels of contaminants.
Plus, its 20x objective allows for closer analysis of morphological information about particles for even more detailed purity analysis.
*Dynabeads refers to the magnetic beads produced by Thermo Fisher Scientific, Inc. Halo Labs is not affiliated with Thermo Fisher Scientific, Inc., and references to Dynabeads or any other third-party trademark do not imply sponsorship, endorsement, or approval.
Featured products
Frequently Asked Questions
Cell therapy manufacturing typically involves several key steps, including:
- 1. Cell Sourcing: Obtaining cells from suitable sources, such as the patient's own cells (autologous) or donor cells (allogeneic).
- 2. Cell Isolation and Expansion: Isolating and expanding the desired cell population in culture under controlled conditions to generate sufficient cell numbers for therapy.
- 3. Genetic Modification (if applicable): Introducing genetic modifications, such as with CAR (chimeric antigen receptor) constructs, to enhance cell therapeutic properties.
- 4. Cell Harvesting: Collecting the cultured cells and preparing them for infusion or further processing.
- 5. Quality Control: Performing rigorous quality control tests to ensure the safety, purity, and potency of the cell therapy product.
- 6. Administration: Administering the cell therapy product to patients via infusion or injection, following strict clinical protocols.
Each step requires careful optimization and validation to ensure product consistency, efficacy, and safety throughout the manufacturing process.
Like many biological therapies, CAR T-cell therapy can be expensive due to the complexity of the treatment process, personalized nature of the therapy, and high manufacturing costs. CAR-T cell therapy involves isolating a patient's T cells, genetically modifying them to target cancer cells, expanding them in culture, and infusing them back into the patient. Additionally, extensive quality control measures, clinical monitoring, and post-treatment care contribute to the overall cost of therapy. However, as research advances and manufacturing processes improve, efforts are being made to reduce the cost of CAR-T cell therapy and increase accessibility to patients.